KEY POINTS
The field of transplantation has made tremendous advances in the last 50 years, mainly due to refinements in surgical technique and development of effective immunosuppressive medications.
Although immunosuppressive medications are essential for transplantation, they are associated with significant short- and long-term morbidity.
Opportunistic infections can be significantly lowered by the use of appropriate antimicrobial agents.
Kidney transplantation represents the treatment of choice for almost all patients with end-stage renal disease. The gap between demand (patients on the waiting list) and supply (available kidneys) continues to widen.
Pancreas transplantation represents the most reliable way to achieve euglycemia in patients with poorly controlled diabetes.
The results of islet transplantation continue to improve but still trail those of pancreas transplantation.
Liver transplantation has become the standard of care for many patients with end-stage liver failure and/or liver cancer.
BACKGROUND
Organ transplantation is a relatively novel field of medicine that has made significant progress since the second half of the twentieth century. Advances in surgical technique and a better understanding of immunology are the two main reasons that transplants have evolved from experimental procedures, just several decades ago, to a widely accepted treatment today for patients with end-stage organ failure. Throughout the world, for a variety of indications, kidney, liver, pancreas, intestine, heart, and lung transplants are now the current standard of care.
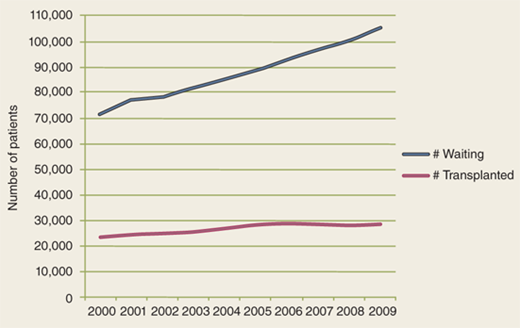
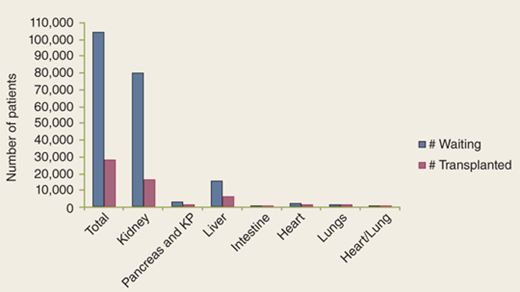
But the success of transplantation has created new challenges. A better understanding of the pathophysiology of end-stage organ failure as well as advances in critical care medicine and in the treatment of various diseases led to expanding the criteria for, and decreasing the contraindications to, transplants. As a result, the discrepancy between the ever-growing number of patients awaiting a transplant and the limited number of organs available is one of the biggest challenges (Fig. 11-1). In 2009 alone, according to the United Network for Organ Sharing (UNOS), about 105,000 patients in the United States were awaiting a transplant, yet the number of transplants performed was only about 28,000 (Fig. 11-2).
DEFINITIONS
In addition to being the overall name of this relatively new field of medicine, transplantation is the process of transferring an organ, tissue, or cell from one place to another. An organ transplant is a surgical procedure in which a failing organ is replaced by a functioning one. The organ is transplanted either orthotopically (implanted in the same anatomic location in the recipient as it was in the donor) or heterotopically (implanted in another anatomic location). Orthotopic transplants require the removal of the diseased organ (heart, lungs, liver, or intestine); in heterotopic transplants, the diseased organ is kept in place (kidney, pancreas).
According to the degree of immunologic similarity between the donor and recipient, transplants are divided into three main categories: (a) An autotransplant is the transfer of cells, tissue, or an organ from one part of the body to another part in the same person, so no immunosuppression is required. This type of transplant includes skin and vein, bone, cartilage, nerve, and islet cell transplants. (b) An allotransplant is the transfer of cells, tissue, or an organ from one person to another of the same species. The immune system of the recipient recognizes the donated organ as a foreign body, so immunosuppression is required in order to avoid rejection. (c) A xenotransplant is the transfer of cells, tissue, or an organ from one organism to another from a different species. To date, animal-to-human transplants are still experimental procedures, given the very complex immunologic and infectious issues that have yet to be solved.
HISTORY
Over the centuries, multiple references to transplantation can be found in the literature. Yet transplantation as a recognized scientific and medical field began to emerge only in the middle of the twentieth century. Two major events preceded the rise of transplantation.
First, the surgical technique of the vascular anastomosis was developed by French surgeon Alexis Carrel.1 This led to increased transplant activity, especially in animal models. Russian surgeon Yu Yu Voronoy was the first to report a series of human-to-human kidney transplants in the 1940s.2 But the outcomes were dismal, mainly because of the lack of understanding of the underlying immunologic processes.
Second, the findings of British scientist Sir Peter B. Medawar in the 1940s were also key.3 In his work with skin grafts in animal models and in human burn patients, he learned that the immune system plays a crucial role in the failure of skin grafts. His research led to a better understanding of the immune system and is considered to be the birth of transplant immunobiology.
The first human transplant with long-term success was performed by Joseph Murray in Boston, Massachusetts, in 1954.4 Because it was a living related kidney transplant between identical twins, no immunosuppression was required; the recipient lived for another 8 years before he died of issues unrelated to the transplanted kidney. Other centers performed similar transplants and could reproduce the good results.
Ultimately, attempts were made to perform kidney transplants between nonidentical individuals. For immunosuppression, total-body radiation and an anticancer agent called 6-mercaptopurine were used; given the profound toxicity of both those methods of immunosuppression, results were discouraging. A breakthrough was achieved in the early 1960s with the introduction of maintenance immunosuppression through a combination of corticosteroids and a less toxic derivative of 6-mercaptopurine, azathioprine.5,6
Increasing experience with kidney transplants and the better results achieved with maintenance immunosuppression paved the way for the era of extrarenal transplants (Table 11-1). In 1963, the first liver transplant was performed by Thomas Starzl in Denver, Colorado, and the first lung transplant was performed by James Hardy in Jackson, Mississippi. In 1966, the first pancreas transplant was performed by William Kelly and Richard Lillehei in Minneapolis, Minnesota. In 1967, the first successful heart transplant was performed by Christiaan Barnard in Cape Town, South Africa. The early years of transplantation were marked by high mortality, mainly because of irreversible rejection. Dramatic changes occurred with the further development of immunosuppression. The groundbreaking event was the introduction of the first anti-T lymphocyte (T cell) drug, cyclosporine, in the early 1980s.7 Since then, with an even better understanding of immunologic processes, many other drugs have been introduced that target specific pathways of rejection. As a result, rejection rates have decreased substantially, allowing a 1-year graft survival rate in excess of 80% in all types of transplants.
ORGAN | YEAR | SURGEON | LOCATION |
|---|---|---|---|
Kidney | 1954 | Joseph E. Murray | Boston, MA |
Liver | 1963 | Thomas E. Starzl | Denver, CO |
Lung | 1963 | James D. Hardy | Jackson, MS |
Pancreas | 1966 | Richard C. Lillehei | Minneapolis, MN |
Heart | 1967 | Christiaan N. Barnard | Cape Town, South Africa |
Small intestine | 1967 | Richard C. Lillehei | Minneapolis, MN |
Heart/lung | 1981 | Bruce Reitz | Stanford, CA |
Multivisceral | 1989 | Thomas E. Starzl | Pittsburgh, PA |
The gradual increase in the organ shortage led to innovative surgical techniques. For example, deceased donor split-liver transplants and living donor liver transplants have helped expand the liver donor pool. Similarly, living donor intestine and pancreas techniques have been developed. The evolution of donor nephrectomy from an open to a minimally invasive procedure (laparoscopic or robotic) has helped increase the pool of living kidney donors.
TRANSPLANT IMMUNOBIOLOGY
The outcomes of early transplants were unsatisfactory. The limiting factor was the lack of understanding of immunologic processes. Irreversible rejection was the reason for graft loss in the vast majority of recipients. A better understanding of transplant immunobiology led to significant improvements in patient and graft survival rates.8,9 The immune system is designed as a defense system to protect the body from foreign pathogens, such as viruses, bacteria, and fungi, but it also acts to reject transplanted cells, tissues, and organs, recognizing them as foreign. It mediates other complex processes as well, such as the body’s response to trauma or to tumor growth. No matter what the pathogen is, the immune system recognizes it as a foreign antigen and triggers a response that eventually leads either to death or to rejection of the pathogen.
TRANSPLANT ANTIGENS
Transplants between genetically nonidentical persons lead to recognition and rejection of the organ by the recipient’s immune system, if no intervention is undertaken. The main antigens responsible for this process are part of the major histocompatibility complex (MHC). In humans, these antigens make up the human leukocyte antigen (HLA) system. The antigen-encoding genes are located on chromosome 6. Two major classes of HLA antigens are recognized. They differ in their structure, function, and tissue distribution. Class I antigens (HLA-A, HLA-B, and HLA-C) are expressed by all nucleated cells. Class II antigens (HLA-DR, HLA-DP, and HLA-DQ) are expressed by antigen-presenting cells (APCs) such as B lymphocytes, dendritic cells, macrophages, and other phagocytic cells.
The principal function of HLA antigens is to present the fragments of foreign proteins to T lymphocytes. This leads to recognition and elimination of the foreign antigen with great specificity. HLA molecules play a crucial role in transplant recipients as well. They can trigger rejection of a graft via two different mechanisms. The most common mechanism is cellular rejection, in which the damage is done by activated T lymphocytes. The process of activation and proliferation is triggered by exposure of T lymphocytes to the donor’s HLA molecules. The other mechanism is humoral rejection, in which the damage is done by circulating antibodies against the donor’s HLA molecules. The donor-specific antibodies can be present either pretransplant, due to previous exposure (because of a previous transplant, pregnancy, blood transfusion, or immunization), or posttransplant. After binding to the donor’s HLA molecules, the complement cascade is activated, leading to cellular lysis.
ALLORECOGNITION AND LYMPHOCYTE ACTIVATION
The immune system of each person is designed to discriminate between self and nonself cells and tissues. This process is called allorecognition, with T cells playing the crucial role. The recognition of foreign HLA antigens by the recipient’s T cells may occur by either a direct or an indirect pathway. Direct recognition occurs when the recipient’s T cells are activated by direct interaction with the donor’s HLA molecules. Indirect recognition occurs when the recipient’s T cells are activated by interaction with APCs that have processed and presented the foreign antigen. The foreign antigen can be shed from the graft into the circulation, or it can be identified by the APCs in the graft itself.
Independent of the pathway of foreign HLA antigen presentation, the ensuing activation of T cells is similar. A two-signal model, T-cell activation begins with the engagement of the T-cell receptor (TCR)/CD3 complex with the foreign molecule. This interaction causes transmission of the signal into the cell, named signal 1. However, this signal alone is not sufficient to activate the T cell. An additional costimulatory signal is required, named signal 2. Two well-characterized costimulatory interactions are the CD40/CD154 and B7/CD28 pathways. The “master switch” is turned on by the interaction of CD40 protein with APCs, along with the interaction of CD154 protein with T cells; this ligation induces the upregulation of other costimulatory molecules. Transmission of signal 1 and signal 2 into the cell nucleus leads to upregulation of the transcription of genes for several cytokines, including the T-cell growth factor interleukin-2 (IL-2). In turn, IL-2 activates a number of pathways, leading to proliferation and differentiation of T cells. Rejection is a result of an attack of activated T cells on the transplanted organ.
Although T-cell activation is the main culprit in rejection, B-cell activation and subsequent antibody production also play a role. After the foreign HLA antigen is processed by B cells, it interacts with activated helper T cells, leading to differentiation of B cells into plasma cells and subsequently to their proliferation and antibody production.
CLINICAL REJECTION
Graft rejection is due to a complex interaction of different parts of the immune system, including B and T lymphocytes, APCs, and cytokines. The end result is graft damage caused by inflammatory injury. According to its onset and pathogenesis, rejection is divided into three main types: hyperacute, acute, and chronic (each described in the following sections).
Hyperacute rejection, a very rapid type of rejection, results in irreversible damage and graft loss within minutes to hours after organ reperfusion. It is triggered by preformed antibodies against the donor’s HLA or ABO blood group antigens. These antibodies activate a series of events that result in diffuse intravascular coagulation, causing ischemic necrosis of the graft. Fortunately, pretransplant blood group typing and cross-matching (in which the donor’s cells are mixed with the recipient’s serum, and then destruction of the cells is observed) have virtually eliminated the incidence of hyperacute rejection.
Acute rejection, the most common type of rejection, usually occurs within a few days or weeks posttransplant. According to the mechanism involved, it is further divided into cellular (T-cell–mediated) rejection, humoral (antibody-mediated) rejection, or a combination of both. The diagnosis is based on the results of biopsies of the transplanted organ, special immunologic stains, and laboratory tests (such as elevated creatinine levels in kidney transplant recipients, elevated liver function values in liver transplant recipients, and elevated levels of glucose, amylase, and lipase in pancreas transplant recipients).
Chronic rejection is a slow type of rejection. It can manifest within the first year posttransplant, but most often progresses gradually over several years. The mechanism is not well understood, but the pathologic changes eventually lead to fibrosis and loss of graft function. With advances in immunosuppression, this relatively rare form of rejection is becoming more common.
CLINICAL IMMUNOSUPPRESSION
A successful transplant is a balance between the recipient’s immune response, the donor’s allograft, and pharmacologic immunosuppression. Immunosuppressive regimens are very important to graft and patient survival posttransplant. Immunosuppression has evolved from the use of azathioprine and steroids in the 1960s and 1970s to the development, in the 1980s, of cyclosporine, which increased allograft survival.10,11 The introduction of tacrolimus and mycophenolate mofetil (MMF) in the 1990s further changed the field of transplantation, enabling a variety of combinations to be used for immunosuppression (Table 11-2).
Immunophilin binders Calcineurin inhibitors Cyclosporine Tacrolimus Noninhibitors of calcineurin Sirolimus Antimetabolites Inhibitors of de novo purine synthesis Azathioprine Mycophenolate mofetil Biologic immunosuppression Polyclonal antibodies Atgam Antithymocyte immunoglobulin Monoclonal antibodies Muromonab-CD3 Basiliximab Belatacept Alemtuzumab Rituximab Bortezomib Eculizumab Other Corticosteroids |
Immunosuppressants usually are used in multidrug regimens, aimed at increasing efficacy by targeting multiple pathways to lower the immune response and to decrease the toxicity of individual agents. Certain regimens may involve withdrawal, avoidance, or minimization of certain classes of drugs. Transplant centers generally institute their immunosuppressive protocols based on experience, risk profiles, cost considerations, and outcomes. Immunosuppression is delivered in two phases: induction (starting immediately posttransplant, when the risk of rejection is highest) and maintenance (usually starting within days posttransplant and continuing for the life of the recipient or graft). Thus, the level of immunosuppression is highest in the first 3 to 6 months posttransplant; during this time, prophylaxis against various bacterial, viral, or even antifungal opportunistic infections is also given.12,13
A conventional immunosuppressive protocol might include (a) induction with anti-T-lymphocyte–depleting or nondepleting antibodies and (b) maintenance with calcineurin inhibitors, antiproliferative agents, and corticosteroids. Characteristics of the most common immunosuppressive agents are listed in Table 11-3.
DRUG | MECHANISM OF ACTION | ADVERSE EFFECTS | CLINICAL USES | DOSAGE |
|---|---|---|---|---|
Cyclosporine (CSA) | Binds to cyclophilin Inhibits calcineurin and IL-2 synthesis | Nephrotoxicity Tremor Hypertension Hirsutism | Improved bioavailability of microemulsion form | Oral dose 5 mg/kg per day (given in two divided doses) |
Tacrolimus (FK506) | Binds to FKBP Inhibits calcineurin and IL-2 synthesis | Nephrotoxicity Hypertension Neurotoxicity GI toxicity (nausea, diarrhea) | Improved patient and graft survival in (liver) primary immunosuppression and rescue therapy Used as mainstay of maintenance protocols | IV 0.015 mg/kg per day as continuous infusion PO 0.05 mg/kg per day (given every 12 h) |
Mycophenolate mofetil | Antimetabolite Inhibits enzyme necessary for de novo purine synthesis | Leukopenia GI toxicity | Effective for primary immunosuppression in combination with tacrolimus | 1 g bid PO |
Sirolimus | Inhibits lymphocyte effects driven by IL-2 receptor | Thrombocytopenia Increased serum cholesterol/LDL Poor wound healing | May allow early withdrawal of steroids and decreased calcineurin doses | 2–4 mg/d, adjusted to trough drug levels |
Corticosteroids | Multiple actions Anti-inflammatory Inhibits lymphokine production | Cushingoid state Glucose intolerance Osteoporosis | Used in induction, maintenance, and treatment of acute rejection | Varies from milligrams to several grams per day Maintenance doses, 5–10 mg/d |
Azathioprine | Antimetabolite Interferes with DNA and RNA synthesis | Thrombocytopenia Neutropenia Liver dysfunction | Used in maintenance protocols or if intolerance to mycophenolate mofetil | 1–3 mg/kg per day for maintenance |
Belatacept | T-cell blocker | Increased risk of bacterial infections | New drug for maintenance immunosuppression in renal transplants only | 5–10 mg/kg per day infusion |
INDUCTION
Induction includes the use of depleting (polyclonal) antibodies or nondepleting antibodies within the first month posttransplant. Studies have shown that induction with antibody regimens may prevent acute rejection, potentially leading to improved graft survival and the use of less maintenance immunosuppression.
Rabbit antithymocyte globulin (Thymoglobulin) is a purified gamma globulin obtained by immunizing rabbits with human thymocytes. Atgam, which has largely been replaced by Thymoglobulin, is a purified gamma globulin obtained by immunizing horses with human thymocytes. These agents contain antibodies to T cells and B lymphocytes (B cells), integrins, and other adhesion molecules, thereby resulting in rapid depletion of peripheral lymphocytes. Typically, the total dose of Thymoglobulin is roughly 6 mg/kg, a dose that has been shown to confer adequate lymphocyte depletion and better allograft survival. Doses of 3 mg/kg may not effectively prevent acute rejection, but more doses and prolonged duration increase the risk of infection and the potential occurrence of lymphoma. Thymoglobulin administration causes a cytokine release syndrome, so premedications (acetaminophen and diphenhydramine) are usually given. The principal side effects of Thymoglobulin include fever, chills, arthralgias, thrombocytopenia, leukopenia, and an increased incidence of a variety of infections.14,15
Basiliximab (Simulect) is an anti-CD25 monoclonal antibody. The alpha subunit of the IL-2 receptor, also known as Tac or CD25, is found exclusively on activated T cells. Blockade of this component by monoclonal antibody selectively prevents IL-2–induced T-cell activation. No lymphocyte depletion occurs with basiliximab; it is not designed to be used to treat acute rejection. Its selectivity in blocking IL-2–mediated responses makes it a powerful induction agent without the added risks of infections, malignancies, or other major side effects. Currently, basiliximab is the only available anti-CD25 monoclonal antibody approved for clinical use. Usually, it is followed by the use of calcineurin inhibitors, corticosteroids, and MMF as maintenance immunosuppression.16
Alemtuzumab (Campath), another anti-CD52 monoclonal antibody, was initially used to treat chronic lymphocytic leukemia. The use of alemtuzumab has grown in the field of transplantation, given its profound lymphocyte-depleting effects. It causes cell death by complement-mediated cytolysis, antibody-mediated cytotoxicity, and apoptosis. One dose alone (30 mg) depletes 99% of lymphocytes. Monocyte recovery can be seen at 3 months posttransplant; B-cell recovery at 12 months; and T-cell recovery, albeit only to 50% of baseline, at 36 months. Alemtuzumab causes a significant cytokine release reaction and often requires premedications (steroids and antihistamines). Because of the long-lasting T-cell depletion, the risks of infection and posttransplant lymphoproliferative disorder remain. Currently, alemtuzumab is available only through a limited distribution program, not through commercial medication distributors.17,18
MAINTENANCE
Corticosteroids have had a role in immunosuppression since the beginning of the field of transplantation. Despite numerous attempts to limit or discontinue their use, they remain an integral component of most immunosuppressive protocols, for both induction and maintenance. Moreover, they are often the first-line agents in the treatment of acute rejection. Steroids bind to glucocorticoid-responsive elements in DNA that prevent the transcription of cytokine genes and cytokine receptors. In addition, steroids have an impact on lymphocyte depletion, on decreases in cell-mediated immunity, and on T-cell activation of many phases of rejection.
Nonetheless, the numerous adverse effects of steroid therapy contribute significantly to morbidity in transplant recipients.19 Common side effects include acne, increased appetite and associated weight gain, mood changes, diabetes, hypertension, and impaired wound healing.
One of the most common maintenance immunosuppressive regimens consists of triple-drug therapy: prednisone, a calcineurin inhibitor, and an antimetabolite. Large doses of steroids are usually given perioperatively and in the immediate postoperative period. Protocols vary by center, but the steroid dose is usually tapered to an adult dose of roughly 5 to 15 mg daily, or completely stopped at some point. Steroids are substrates for CYP3A4, CYP3A5, and P-glycoprotein pathways where drug interactions might need to be monitored.20,21
An antimetabolite, azathioprine (AZA) is converted to 6-mercaptopurine and inhibits both the de novo purine synthesis and salvage purine synthesis. AZA decreases T-lymphocyte activity and decreases antibody production. It has been used in transplant recipients for more than 40 years, but became an adjunctive agent after the introduction of cyclosporine. With the development of newer agents such as MMF, the use of AZA has decreased significantly. However, it is preferred in recipients who are considering conceiving a child, because MMF is teratogenic in females and can cause birth defects. AZA might be an option for recipients who cannot tolerate the gastrointestinal (GI) side effects of MMF.
The most significant side effect of AZA, often dose-related, is bone marrow suppression. Leukopenia is often reversible with dose reduction or temporary cessation of the drug. Other significant side effects include hepatotoxicity, pancreatitis, neoplasia, anemia, and pulmonary fibrosis. Its most significant drug interaction is with allopurinol, which blocks AZA’s metabolism, increasing the risk of pancytopenia. Recommendations are to not use AZA and allopurinol together, or if doing so is unavoidable, to decrease the dose of AZA by 75%.22
Approved in May 1995 by the U.S. Food and Drug Administration (FDA) for preventing acute rejection after kidney transplants, MMF has now been incorporated into routine maintenance regimens after many solid organ transplants. Mycophenolate is the prodrug of mycophenolate acid, derived from Penicillium fungi. Mycophenolate acid is an inhibitor of inosine monophosphate dehydrogenase (IMPDH) involved in the de novo pathway of purine synthesis.23 MMF is available in capsules (250 and 500 mg); the starting dose is 1 g twice daily. In hopes of decreasing the GI side effects, an enteric-coated formulation called Myfortic was developed; its benefits have not been clearly demonstrated in studies, but in some conversion studies, patients did report less GI intolerance. The pharmacokinetics of MMF are complex; mycophenolic acid (MPA) levels are not routinely performed at most transplant centers. Studies have shown that MPA levels and the incidence of rejection are not significantly correlated.24 The most common side effects of MMF are GI in nature, most commonly diarrhea, nausea, dyspepsia, and bloating. Esophagitis and gastritis occur in roughly 5% of recipients and may represent a cytomegalovirus (CMV) or herpesvirus family infection. The other important side effects are leukopenia, anemia, and thrombocytopenia (Table 11-4). Leukopenia can sometimes be reversed by lowering the MMF dose and discontinuing other agents like valganciclovir. MMF does not have any significant drug interactions, but clinicians should be careful to avoid additive toxicities with other medications that might lead to leukopenia and thrombocytopenia.
COMMON SIDE EFFECTS | OTHER MEDICATIONS THAT INCREASE BLOOD LEVELS | OTHER MEDICATIONS THAT DECREASE BLOOD LEVELS | OTHER MEDICATIONS THAT POTENTIATE TOXICITY | |
|---|---|---|---|---|
Cyclosporine (CSA) | Hypertension, nephrotoxicity, hirsutism, neurotoxicity, gingival hyperplasia, hypomagnesemia, hyperkalemia | Verapamil, diltiazem, clarithromycin, azithromycin, erythromycin, azole antifungals, protease inhibitors, grapefruit juice | Isoniazid, carbamazepine, phenobarbital, phenytoin, rifampin, St. John’s Wort | Nephrotoxicity: ganciclovir, aminoglycosides, NSAIDs, ACE-Is, and ARBs |
Tacrolimus (FK506) | Hypertension, nephrotoxicity, alopecia, hyperglycemia, neurotoxicity, hypomagnesemia, hyperkalemia | Verapamil, diltiazem, clarithromycin, azithromycin, erythromycin, azole antifungals, protease inhibitors, grapefruit juice | Isoniazid, carbamazepine, phenobarbital, phenytoin, rifampin, St. John’s wort | Nephrotoxicity: ganciclovir, aminoglycosides, NSAIDs, ACE-Is, and ARBs |
Sirolimus | Thrombocytopenia and neutropenia, elevated cholesterol, extremity edema, impaired wound healing | Verapamil, diltiazem, clarithromycin, azithromycin, erythromycin, azole antifungals, protease inhibitors, grapefruit juice | Isoniazid, carbamazepine, phenobarbital, phenytoin, rifampin, St. John’s wort | — |
Mycophenolate mofetil | Leukopenia, thrombocytopenia, GI upset | — | Cholestyramine, antacids | Bone marrow suppression: valganciclovir, ganciclovir, TMP-SMX |
Corticosteroids | Hyperglycemia, osteoporosis, cataracts, myopathy, weight gain | — | — | — |
Azathioprine | Leukopenia, anemia, thrombocytopenia, neoplasia, hepatitis, cholestasis | — | — | Bone marrow suppression: allopurinol, sulfonamides |
The first mammalian target of rapamycin (mTOR) inhibitors to enter clinical use was sirolimus (Rapamune). A key regulatory kinase, mTOR changes cells from the G1 to S phase in the cell cycle, in response to proliferation signals provided by cytokines like IL-2. The mTOR inhibitors bind to FK506-binding protein (FKBP), and the sirolimus-FKBP complex binds to mTOR. Sirolimus also inhibits proliferation of vascular smooth muscle cells, possibly easing the vasculopathy and progressive fibrosis that can affect allografts. Sirolimus is a substrate for CYP3A4/4 and has many significant drug interactions (see Table 11-4).
To date, sirolimus has been used in a variety of combinations for maintenance immunosuppression, alone or in conjunction with one of the calcineurin inhibitors. In such combinations, sirolimus usually is used to help withdraw, or completely avoid the use of, steroids. It also has been used as an alternative to tacrolimus or cyclosporine, in a calcineurin-sparing protocol. One of the most significant side effects of sirolimus is hypertriglyceridemia, a condition that may be resistant to statins and fibrates. Impaired wound healing (immediately posttransplant in particular), thrombocytopenia, leukopenia, and anemia also are associated with sirolimus, and these problems are exacerbated when it is used in combination with MMF.25,26
The introduction of cyclosporine in the early 1980s dramatically altered the field of transplantation by significantly improving outcomes after kidney transplantation. Cyclosporine binds with its cytoplasmic receptor protein, cyclophilin, which subsequently inhibits the activity of calcineurin, thereby decreasing the expression of several critical T-cell activation genes, the most important being for IL-2. As a result, T-cell activation is suppressed.27
Many formulations of cyclosporine exist, so it is important to know which one the transplant recipient is taking. Sandimmune, an older, oil-based formulation, has poor bioavailability and variable absorption. The newer formulations, Gengraf and Neoral, are microemulsified with improved bioavailability. Cyclosporine can be given intravenously or orally to maintain trough levels of 250 to 350 ng/mL for the first 3 months posttransplant; then it can be tapered to 150 to 250 ng/mL.28
The metabolism of cyclosporine is via the cytochrome P450 system, resulting in many significant drug interactions (see Table 11-4). Calcineurin inhibitors are nephrotoxic and constrict the afferent arteriole in a dose-dependent, reversible manner (Table 11-5). They also can cause hyperkalemia and hypomagnesemia. Several neurologic complications, including headaches, tremor, and seizures, also have been reported.29
INTERACTIONS | MEDICATIONS |
|---|---|
Inhibition of metabolism | Clarithromycin, erythromycin, azole antifungals, diltiazem, verapamil, nicardipine, amiodarone, grapefruit juice, ritonavir, azithromycin |
Induction of metabolism | Nevirapine, rifampin, St. John’s wort, carbamazepine, phenobarbital, phenytoin, caspofungin |
Hyperkalemia | Potassium-sparing diuretics, angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor blockers (ARBs), β-blockers, trimethoprim-sulfamethoxazole |
Nephrotoxicity | Nonsteroidal anti-inflammatory drugs, aminoglycosides, amphotericin, ACE-Is, ARBs |
Cyclosporine has several undesirable cosmetic effects, including hirsutism and gingival hyperplasia. It is associated with a higher incidence of hypertension and hyperlipidemia than is tacrolimus.
The calcineurin inhibitor tacrolimus (Prograf) is now the backbone of most immunosuppressive regimens. Tacrolimus acts by binding FKBPs, causing roughly 10 to 100 times more potent inhibition of IL-2 production than cyclosporine (which acts by binding cyclophilins). It can be given intravenously, orally, or sublingually to maintain trough levels of 8 to 12 ng/mL for the first 3 months posttransplant; then it can be tapered to 6 to 10 ng/mL.
The metabolism of tacrolimus is via the cytochrome P450 system, resulting in many significant drug interactions (see Table 11-4).
Tacrolimus causes a higher incidence of new-onset diabetes posttransplant than does cyclosporine. Other side effects include alopecia, nephrotoxicity, neurotoxicity, hypertension, hyperkalemia, hypomagnesemia, and an increased incidence of certain types of infection.30
The best-characterized pathway of T-cell costimulation includes CD28; its homologue, the cytotoxic T-lymphocyte–associated protein 4 (CTLA4); and their ligands, CD80 and CD86. Belatacept (also known as LEA29Y) was developed through two amino acid substitutions to abatacept (also known as CTLA4-Ig), a fusion protein consisting of the extracellular domain of CTLA4 and the Fc domain of immunoglobulin G (IgG). It is a high-avidity molecule with slower dissociation rates.
Recent trials have compared the use of belatacept vs. a standard cyclosporine protocol in recipients of living donor, deceased donor, and extended-criteria donor kidneys. Belatacept was not inferior to cyclosporine in both patient and allograft survival rates, but was associated with a higher rate of biopsy-proven acute cellular rejection.
In terms of adverse effects, belatacept differs from standard calcineurin-based regimens because of an increased risk of posttransplant lymphoproliferative disorder (PTLD); the greatest risk is in recipients who are Epstein-Barr virus (EBV)-seronegative pretransplant. The FDA recommends the use of belatacept only in seropositive recipients. Studies in liver transplant recipients were halted early because of increased mortality rates.
However, belatacept does have a lower incidence of cardiovascular risk factors including metabolic lipid disorders, hypertension, neurotoxicity, glucose abnormalities, and adverse cosmetic effects. Except for the increased risk of malignancy, the more favorable adverse effect profile of belatacept and its convenient monthly dosing schedule may make it an attractive option for maintenance of immunosuppression, possibly improving compliance.31,32
HUMORAL REJECTION
A chimeric anti-CD20 (anti-B cell) monoclonal antibody,rituximab is currently FDA approved for treating lymphoma. The CD20 antigen is expressed early in the B-cell cycle but is absent on mature plasma cells. The variable region binds to CD20 through three different mechanisms: (a) antibody-dependent cell cytotoxicity, (b) complement-dependent cell killing, and (c) induction of apoptotic cell death. The use of rituximab has grown to include the treatment of antibody-mediated rejection and use in desensitization protocols. Studies so far have been small, with rituximab usually used in conjunction with plasmapheresis, steroids, and intravenous immunoglobulin (IVIG).33,34,35
A proteasome inhibitor, bortezomib is FDA approved for treating multiple myeloma. It can directly target plasma cells. Traditional treatments have been successful in removing antibodies, inhibiting antibody activity, or lowering antibody production; however, targeting mature antibody production in plasma cells has not met with success. Bortezomib has been shown to cause apoptosis of normal plasma cells, thereby decreasing alloantibody production in sensitized patients. Several case reports and series have described the use of bortezomib for the treatment of antibody-mediated rejection and in desensitization protocols.34,36,37
A humanized monoclonal antibody with high affinity for C5, eculizumab is a first-in-class, FDA-approved agent for treating paroxysmal nocturnal hemoglobinuria and hemolytic uremic syndrome. It blocks the activation of the terminal complement cascade. Most antibody-mediated rejection episodes are associated with early complement activation as evidenced on renal transplant biopsies by the presence of C4d+ staining of the peritubular capillaries. Given its highly selective mechanism of action, this agent is predicted to be useful to treat antibody-mediated rejection and to desensitize patients pretransplant. However, its serious adverse effects include an increased risk of infections, especially due to encapsulated bacteria such as Neisseria meningitidis. Patients should be immunized with meningococcal vaccine at least 2 weeks before the administration of eculizumab.34,38,39
INFECTIONS AND MALIGNANCIES
Advances in immunosuppression have led to improved graft survival rates. However, the growing population of immunosuppressed patients, in turn, has led to an increased incidence of opportunistic infections and malignancies. Such posttransplant complications have become important barriers to long-term disease-free survival.
Transplant recipients are predisposed to a variety of infections. Immunosuppression is the obvious reason. Moreover, such patients have already endured end-stage organ disease pretransplant and then the stress of an invasive transplant operation. Posttransplant, they continue to have significant comorbid conditions.
Early infections (i.e., infections occurring within 1 month posttransplant) can be due to a wide spectrum of pathogens (bacterial, viral, and fungal). In the immediate postoperative period, recipients are significantly compromised from the stress of the operation, from induction immunosuppression, and often from initially impaired graft function. Infections during this period can be devastating.
It is imperative to differentiate between medical and surgical infections. Surgical infections are the most common and require expedient surgical intervention. Typical examples include generalized peritonitis, intra-abdominal abscesses, and wound infections.
In liver and pancreas recipients, surgical infections are most severe. The incidence of intra-abdominal infections is decreasing, but they remain a significant problem: they are the second most common reason (after vascular thrombosis) for graft loss in pancreas recipients.
Lengthy operations with significant blood loss, prolonged warm and cold ischemic times, and spillage of contaminated fluid (bile, urine, or bowel contents) predispose patients to intra-abdominal infections. Other prominent risk factors are the high level of induction immunosuppression immediately posttransplant and anastomotic leaks. Furthermore, pretransplant infections can re-emerge or worsen.
The signs and symptoms of intra-abdominal infections are those of peritonitis: fever, hypotension, ileus, and abdominal pain, although the latter can be masked by immunosuppression. Treatment entails a prompt return to the operating room. Intra-abdominal infections are usually polymicrobial, involving several bacterial and fungal species. Common bacterial isolates include Escherichia coli, as well as Enterococcus, Klebsiella, and Pseudomonas species. Common fungal isolates are Candida albicans, Candida krusei, and Candida glabrata. Localized infections or abscesses can be treated with percutaneous drainage and antibiotics.
Medical infections include respiratory, urinary tract, and bloodstream infections. Medical treatment should also be aggressive, often including empiric antibiotics and antifungal medications even before culture results are available. Recipients of organs from infected donors should be treated per the results of donor culture speciation and the antibiotic sensitivity profile.
Late infections primarily are due to chronic immunosuppression, specifically the depression of cell-mediated immunity that renders recipients susceptible to viruses, fungi, and parasites.
Members of the herpesvirus group are the most common etiologic agents of viral infections posttransplantation, with herpes simplex virus (HSV), CMV, and EBV being the most prominent. Pretransplant exposure to viruses may confer immunity. Recipients who are seronegative for HSV, CMV, and/or EBV have a higher incidence of posttransplant infections, especially if they receive donor allografts from seropositive donors.
CMV is a latent infection that can be transmitted to seronaive recipients by donor organs from seropositive individuals, can reactivate during immunosuppression, or both. Infections usually occur 3 to 6 months posttransplant or during treatment for rejection. The incidence of CMV has been greatly reduced with 12-week acyclovir prophylaxis.40 CMV infections range from an asymptomatic or mild flu-like syndrome to tissue-invasive disease resulting in pneumonitis, hepatitis, and GI ulcerations. Symptomatic infections and all tissue-invasive CMV disease should be treated with intravenous (IV) ganciclovir, a reduction in immunosuppression, or both, although successful treatment of mild to moderate rejection and concurrent mild to moderate CMV disease has been described.
EBV infections range from a mild mononucleosis syndrome to severe hepatitis and highly morbid PTLD. PTLD ranges from a localized tumor to a progressive, diffuse infiltration of various organs including the brain. It results from the proliferation of EBV-positive B cells in immunosuppressed patients. The main risk factors are a high degree of immunosuppression and a predisposing EBV serostatus (seronaive recipient, seropositive donor). Among patients with early lesions, the first line of treatment is to reduce immunosuppression. For those with more advanced PTLD, rituximab is used.
After 6 months posttransplant, the risk of invasive fungal infections is closely associated with environmental exposures. Blastomyces dermatitidis grows in moist soil in the Midwest and Southeast regions of the United States. Diagnosis is confirmed by biopsy; the preferred treatment is IV amphotericin B.
Coccidioides immitis can cause invasive coccidioidomycosis after inhalation of aerosolized infectious particles. It is endemic in the Southwest, Northern Mexico, and various parts of Central and South America. This infection can be resilient and difficult to treat. The first line of treatment is high-dose amphotericin B.
Histoplasma capsulatum is found in chicken and bat droppings in the Ohio River and Mississippi River valleys. Dissemination is commonplace; up to a quarter of patients have central nervous system (CNS) involvement. Treatment consists of prolonged (3 to 13 months) administration of oral itraconazole.
Opportunistic infections with Aspergillus, Cryptococcus, Mucor, and Rhizopus species are rare but can cause serious infections. Patients with invasive Candida or Aspergillus infections have a 20% mortality rate. Prophylaxis with fluconazole has been shown to reduce invasive fungal infections in liver recipients.41
Pneumocystis jiroveci (also known as PCP) is ubiquitous and can cause pulmonary disease in immunocompromised patients. However, trimethoprim-sulfamethoxazole (TMP-SMX) is effective prophylaxis against PCP, and daily, lifelong administration has virtually eliminated this infection among transplant recipients.
Chronic immunosuppression increases the risk of developing certain types of malignancies. The most extensive data, from a cohort study involving more than 175,000 solid organ transplant recipients, showed that 10,656 of them developed malignancies. The standardized incidence ratio was 2.10 (as compared with the general population). Recipients had at least a fivefold increase (as compared with the general population) in these types of malignancies: Kaposi’s sarcoma, nonmelanoma skin cancer, non-Hodgkin’s lymphoma, and cancer of the liver, anus, vulva, and lip. In addition, recipients had a statistically significant increase (as compared with the general population) in melanoma, Hodgkin’s lymphoma, and cancer of the lung, kidney, colon, rectum, and pancreas.42
ORGAN PROCUREMENT AND PRESERVATION
Organ procurement is a key element in organ transplantation. Currently, 58 organ procurement organizations (OPOs) exist in the United States, all members of the Organ Procurement and Transplantation Network (OPTN), which is a federally mandated network created by and overseen by UNOS. Each OPO is responsible for evaluating and procuring deceased donor organs for transplantation in a specific geographic region. Hospitals receiving any type of federal reimbursement for their services (whether transplant-related or not) are required to report all deaths to their OPO in a timely manner. Each OPO then determines the medical suitability of the deceased for organ donation; requests consent for donation from family members; if consent is given, contacts the OPTN to analyze and identify potential recipients whose HLA antigens most closely match those of the donor; and arranges for the recovery and transport of any donated organs.
Strategies to increase organ donation and utilization have been successfully implemented in the last 10 years. The nationwide “Organ Donation Breakthrough Collaborative,” sponsored by the U.S. Department of Health and Human Services in 2003, brought the OPOs and transplant communities into a single concerted program to develop best practices guidelines. However, a severe donor shortage remains. The number of living organ donors peaked in 2007 and has declined since.
Alternative options include tissue engineering and stem cell research, but those fields are in their infancy in terms of producing fully functional and vascularized human organs. With the development of genetic knockout pigs, xenotransplantation still shows promise, but two problems in particular—immunologic barriers and xenosis (also known as zoonosis) of endogenous porcine retroviruses—have yet to be satisfactorily addressed.
Today, the gap between patients waiting for organ transplants and the number of organs available continues to widen. More than 110,000 patients are on the waiting list for solid organ transplants, but only 28,456 transplants were performed in 2011.
Most transplants today utilize organs from deceased donors. Formerly, death was determined by the cessation of both cardiac and respiratory function.
In 1968, the concept of “irreversible coma” was introduced by an ad hoc committee report at Harvard Medical School; that concept was pivotal to the final acceptance, in 1981, of “brain death” as a legal definition in the United States. The legal language states that the declaration of brain death should be in accordance with acceptable medical standards, but does not specify clinical methodology. It is customary for hospitals to establish their own policies to declare brain death, according to their standards of care and local regulations.
Typically, brain death is defined as the irreversible cessation of brain function, including the brainstem. The presence of medical conditions that mimic brain death—such as drug overdose, medication side effects, severe hypothermia, hypoglycemia, induced coma, and chronic vegetative state—need to be excluded. The latest evidence-based guideline on determining brain death in adults reaffirmed the validity of current clinical practice.43 Briefly, the clinical diagnosis of brain death consists of four essential steps: (a) establishment of the proximate cause of the neurologic insult; (b) clinical examinations to determine coma, absence of brainstem reflexes, and apnea; (c) utilization of ancillary tests, such as electroencephalography (EEG), cerebral angiography, or nuclear scans, in patients who do not meet clinical criteria; and (d) appropriate documentation. A similar guideline on determining brain death in pediatric patients was recently developed.44
Once the diagnosis of brain death has been established, the local OPO assumes the care of the potential donor and initiates the process of donor evaluation and organ donation, and the potential donor is screened for contraindications to donation. The medical history and social history are obtained from the available family members. A battery of tests, including serologic or molecular detection of human immunodeficiency virus (HIV) and viral hepatitis, are performed. The exact medical conditions that preclude donation vary; nonetheless, in the United States, infections and other medical conditions that determine eligibility are dictated by UNOS bylaws and routinely reviewed and updated.
The OPO focuses on preserving organ function and optimizing peripheral oxygen delivery until organ procurement commences.45 In all deceased donors, core temperature, systemic arterial blood pressure, arterial oxygen saturation, and urine output must be determined routinely and frequently. Arterial blood gases, serum electrolytes, blood urea nitrogen, serum creatinine, liver enzyme, hemoglobin, and coagulation tests need to be monitored regularly. In all brain-dead donors, elevated intracranial pressure triggers a compensatory catecholamine response to maintain cerebral profusion pressure. Ischemic injury to the spinal cord and the sympathetic system may lead to a profound vasodilation. As a result, brain-dead donors frequently have severe hemodynamic and metabolic derangements, so aggressive monitoring and intervention are required to prevent loss of precious organs.
Previous studies of deceased donor care focused on organ-specific resuscitation protocols that resulted in only marginal gains in the number of organs transplanted. The latest developments center on multisystem protocols to increase the number of organs transplanted per donor (OTPD).46,47 The goals are to maintain a core temperature between 36.0 and 37.5°C, a mean arterial pressure >70 mmHg or a systolic pressure >100 mmHg, and a hemoglobin level between 7 and 10 g/dL; hormonal therapy and aggressive treatment of arrhythmias and metabolic derangements are also called for.47
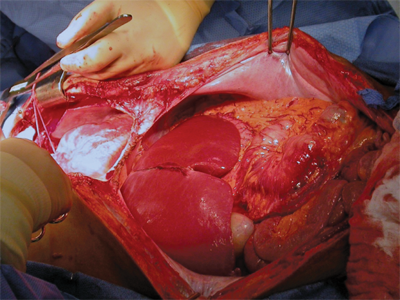
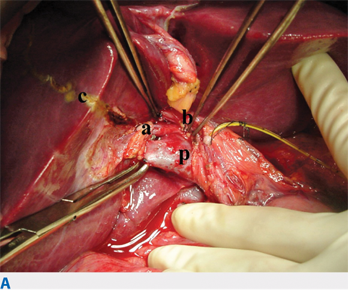
Procurement of multiple organs (heart, lungs, kidney, liver, pancreas, and/or small bowel), or multivisceral procurement, was first described by the Pittsburgh group in 1987.48 Since then, most centers have incorporated changes, especially with regard to the timing and location of dissection and flushing.49,50 The basic steps involve a long incision to provide wide exposure of all thoracic and abdominal organs (Fig. 11-3). A Cattell-Braasch maneuver (complete mobilization of the distal small bowel, right colon, and duodenum) is performed to allow for identification of the distal aorta, iliac bifurcation, and distal inferior vena cava (IVC). The infrarenal aorta is the site for inserting the cannula that will allow for flushing of the organs with cold preservation solution. Sometimes, division of the inferior mesenteric artery is necessary to facilitate the exposure of the distal aorta. The third portion of the duodenum is retracted cephalad to expose the root of the superior mesenteric artery (SMA). Limited dissection is performed at the root of the SMA, which is encircled with a vessel loop to enable its temporary occlusion at the time of flushing, thus reducing the incidence of overperfusion injury to the pancreas.
A large anomalous or replaced right hepatic artery typically rises from the SMA, and this should be identified and preserved. Lateral to the SMA is the inferior mesenteric vein (IMV), which can be cannulated for portal flushing. Dissection of the hepatic hilum and the pancreas should be limited to the common hepatic artery (CHA), and branches of the CHA (e.g., splenic, left gastric, and gastroduodenal arteries) are exposed. The gastrohepatic ligament is carefully examined to preserve a large anomalous or replaced left hepatic artery, if present. The supraceliac aorta can be exposed by dividing the left triangular ligament of the liver and the gastrohepatic ligament.
The common bile duct is transected at the superior margin of the head of the pancreas. The gallbladder is incised and flushed with ice-cold saline to clear the bile and sludge. If the pancreas is to be procured, the duodenum is flushed with antimicrobial solution. Before the cannulation of the distal aorta, systemic heparinization (300 units/kg) is administered. The supraceliac aorta is clamped; cold preservation fluid is infused via the aortic (systemic) and IMV (portal) cannulas. The thoracic organs, liver, pancreas, and kidneys are then removed.
Given the severe shortage of donor organs, donation after cardiac death (DCD)—also known as donation by non–heart-beating donors (NHBDs)—was reintroduced to the transplant community in the 1990s.51 The category of DCD (Maastricht classification) was initially proposed at an international workshop and is now widely adopted for organ procurement.52 Currently, most NHBDs in the United States meet Maastricht classification III; that is, they have suffered a devastating injury with no chance of a meaningful recovery but do not meet the criteria for brain death. After consent for donation is obtained from the next of kin, the donor’s life support is removed. After the cessation of cardiac and respiratory function, organ procurement commences. DCD procurement protocols vary between states; religious and cultural differences need to be taken into consideration. The surgical team must be familiar with, and respect, the local protocol.
With cardiac death (as opposed to brain death), warm ischemic injury to organs can occur during the period between circulatory cessation and rapid core cooling through perfusion of preservation solution. However, the difference in long-term outcomes is negligible for recipients of organs from either type of donor. Still, a significant percentage of liver grafts procured after cardiac death, especially those with more than 25 minutes of warm ischemic time, develop devastating ischemic cholangiopathy and fail.53
A new development to minimize ischemic injury to organs procured after cardiac death has been the application of extracorporeal membrane oxygenation (ECMO). With ECMO, DCD differs in two key ways: (a) cannulation occurs before withdrawal of life support and (b) organs are perfused via ECMO with warm oxygenated blood after declaration of cardiac death. The initial experience with organs procured using ECMO has been encouraging.
Surgeons who perform multiple organ retrieval should be familiar and experienced with the super-rapid technique described by the Pittsburgh group.54 Preferably, NHBDs undergo withdrawal of life support in the operating room after the surgical site is prepped and draped, as soon as the surgical team is ready. Alternatively, the NHBD is transported to the operating room after declaration of cardiac death.
A midline incision is used to rapidly gain entry into the abdominal cavity. An assistant retracts the small bowel and the sigmoid colon laterally, so that the bifurcation of the aorta can be easily identified on the left side of the vertebral column. A short segment of the distal aorta is dissected out from the retroperitoneum. A moist umbilical tape is passed around the aorta, which is used to secure a cannula. The distal aorta is clamped. Next, a cannula is passed cephalad through an aortotomy and secured. Flushing with cold preservation solution is started at once, followed by cross-clamping the aorta proximally (thoracic aorta) and venting through the vena cava. The portal flush is then instituted.
The rest of the procedure is similar to procurement after brain death, with two noticeable differences. First, to avoid injury to a large anomalous or replaced left hepatic artery, the gastrohepatic ligament and the left gastric artery are separated from the stomach at the lesser curvature. Second, to avoid injury to a large anomalous or replaced right hepatic artery, the SMA is examined before it is divided. If the pancreas is not procured, a common aortic patch encompassing both the SMA and the celiac artery can be procured with the liver.
The maxim of medical ethics is “primum non nocere” (above all, do no harm), and for that reason, living organ donation presents unique ethical and legal challenges. Performing potentially harmful operations to remove organs from healthy individuals seems, at first glance, to contradict that maxim. But in fact, the ethical framework of living organ donation rests on three guiding principles respected in all discussions of medical practice: beneficence to the recipient, nonmaleficence to the donor, and the donor’s right to autonomy.55 In order to achieve optimal outcomes (the common good), transplant professionals should focus on maximizing the benefits for the recipient and minimizing the damage to the donor. The Uniform Anatomical Gift Act adopted by all states in the United States (with slight variations) provides the legal framework for competent adult living donors to decide whether or not to donate. It is the fiduciary duty of transplant professionals to explain the risks of organ donation. Any decision to donate should be uncoerced, and no enticements should be offered.
The use of living donors offers numerous advantages for recipients in need. First and foremost is the availability of lifesaving organs for those who would otherwise succumb to the progression of their end-stage disease. In certain parts of the world, such as East Asia, the concept of brain death and the use of deceased donors conflict with the prevailing culture or religion. Even in countries where the use of deceased donors is accepted, the use of living donors may significantly shorten the waiting time for recipients. A shorter waiting time generally implies a healthier recipient—one whose body has not been ravaged by prolonged end-stage organ failure. Moreover, with the use of living donors, transplants are planned (rather than emergency) procedures, allowing for better preoperative preparation of the recipient. Receiving an organ from a closely matched relative may also have immunologic benefits. And long-term results may be superior with the use of living donors, as is certainly the case with kidney transplants.
The major disadvantage is the risk to the living donor. Medically, there is no possibility of benefit to the donor, only the potential for harm. The risk of death associated with donation depends on the organ being removed. For a nephrectomy, the estimated mortality risk is less than 0.05%; for a partial hepatectomy, about 0.2%. The risk of surgical and medical complications also depends on the procedure being performed. In addition, long-term complications may be associated with a partial loss of organ function after donation. The guiding principle should be minimization of risk to the donor. All potential risks must be carefully explained to the potential donor, and written informed consent must be obtained.56
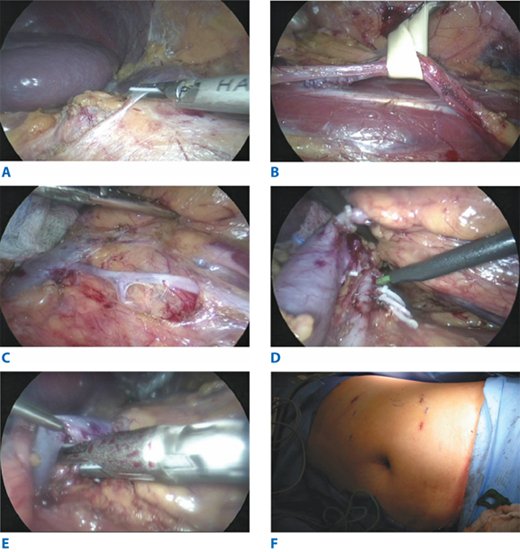
The kidney, the first organ to be transplanted from living donors, is still the most common organ donated by these individuals. The donor’s left kidney is usually preferable because of the long vascular pedicle. Use of living donor kidneys with multiple renal arteries should be avoided, in order to decrease the complexity of the vascular reconstruction and to help avoid graft thrombosis. Most donor nephrectomies are now performed via minimally invasive techniques, that is, laparoscopically, whether hand-assisted or not. With laparoscopic techniques, an intraperitoneal approach is most common: it involves mobilizing the colon, isolating the ureter and renal vessels, mobilizing the kidney, dividing the renal vessels and the distal ureter[C6], and removing the kidney (Fig. 11-4). Extensive dissection around the ureter should be avoided, and the surgeon should strive to preserve as much length of the renal artery and vein as possible.
Figure 11-4.
Laparoscopic left donor nephroureterectomy. A. Takedown of splenic flexure of colon to expose the left renal hilum. B. Dissection of left ureter off the psoas muscle. C. Dissection of left renal vein and gonadal vein. Left ureter seen lateral to the dissection. D. Dissection of left renal artery. Lumbar veins clipped and divided. E. Endo-TA stapler transection of the left renal artery. F. Placement of ports and Pfannenstiel incision for the donor kidney extraction.
Liver transplants with living donors are not as commonly performed, given the significantly higher rates of donor mortality and morbidity. Initially, only adult donors for pediatric recipients were selected, but now, living donor liver transplants also involve adult donors for adult recipients. In dual graft living donor liver transplants, segmental grafts from two living donors augment the recipient’s graft size.57 The donor hepatectomy is similar to a major lobar hepatectomy, except that it is important to preserve the integrity of the vascular structure until graft resection (Fig. 11-5).
Figure 11-5.
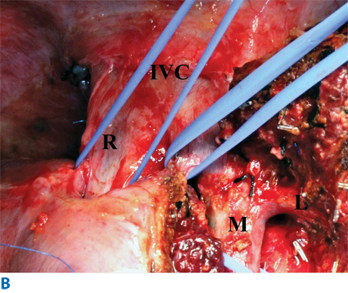
Donor hepatectomy (right hepatectomy). A. The liver parenchymal transection line (c, the Cantlie line) marked with cautery. Right portal vein (p) and right hepatic artery (a) isolated. b = bile duct. Cystic duct was cannulated for intraoperative cholangiography. B. Exposure of hepatic veins after transection of the parenchyma. IVC = inferior vena cava; L = left hepatic vein; M = middle hepatic vein; R = right hepatic vein
Living donor transplants of organs other than the kidney and liver are fairly uncommon, but certain centers do perform such transplants. Living donor pancreas transplants involve performing a distal pancreatectomy, with the graft consisting of the body and tail of the pancreas; vascular inflow and outflow are provided by the splenic artery and splenic vein. Living donor intestinal transplants usually involve removal of about 200 cm of the donor’s ileum, with inflow and outflow provided by the ileocolic vessels. Living donor lung transplants involve removal of one lobe of one lung from each of two donors; both grafts are then transplanted into the recipient.
The development and continuing refinement of organ preservation methods have completely revolutionized the transplant field. Extending the time that organs can be safely stored after procurement has enabled better organ utilization and better recipient outcomes.58,59 Hypothermia and pharmacologic inhibition are the two most frequent methods. Both slow—yet cannot completely shut down—the removed organ’s metabolic activity, so both have adverse effects, such as cellular swelling and degradation. Cold storage solutions were introduced to mitigate some of the adverse effects of hypothermia or pharmacologic inhibition alone. Such solutions help prevent cellular swelling and the loss of cellular potassium.
One, and perhaps the most effective, preservation solution was developed at the University of Wisconsin and remains in wide use.60 Its ingredients include lactobionate (which helps prevent cellular swelling and reperfusion injury), raffinose, and hydroxyethyl starch (which helps reduce swelling of endothelial cells, thereby decreasing edema). Histidine-tryptophan-ketoglutarate solution is also currently in wide use.61
Despite enhancements in preservation methods, the amount of time that an organ can be safely stored remains relatively short (hours, not days), particularly with organs from marginal donors. Among kidney recipients, delayed graft function becomes significantly more frequent after cold ischemic times of more than 24 hours, necessitating temporary dialysis, which is associated with increased risks of graft loss and higher costs.62 Among liver recipients, primary nonfunction and biliary complications ensue after prolonged cold ischemic times. In the case of heart and lung recipients, ischemic times should be under 6 hours. All of those times assume the use of normal donors.
There is revived interest in the use of the pulsatile perfusion pump, a kidney graft preservation method that has been available for more than 40 years.63 With the increasing shortage of available donor organs and the rise in the use of organs after cardiac death, the pulsatile perfusion pump is garnering renewed enthusiasm as an adjunct method of preservation, even for donor organs other than kidneys.64,65
KIDNEY TRANSPLANTATION
Ullman reported the first attempted human kidney transplant in 1902.66 For the next 50 years, sporadic attempts all ended in either technical failure or in graft failure from rejection. Joseph Murray performed the first successful kidney transplant in 1954, an epochal event in the history of organ transplantation. In that first case, the immunologic barrier was circumvented by transplanting a kidney between identical twins.67 For his pivotal contribution, Murray shared the Nobel Prize in Physiology or Medicine in 1990 with E. Donnall Thomas for their discoveries concerning “organ and cell transplantation in the treatment of human disease.”
The introduction of AZA (Imuran) in 1960 marked the beginning of a new era in kidney transplantation. With the combination of steroids and AZA for maintenance immunosuppression, the 1-year graft survival rate with a living related donor kidney approached 80%; with a deceased donor kidney, the rate was 65%.68 In the ensuing years, major milestones included the introduction of more effective immunosuppressive medications with lower toxicity profiles, such as polyclonal antilymphocyte globulin in the 1970s, cyclosporine in the 1980s, tacrolimus in the 1990s, and biologics in the first decade of the twenty-first century, as previously mentioned.
Parallel to the developments in medical science were the transplant community’s concerted efforts to improve use of healthcare resources. In the United States, the Social Security amendments of 1972 provided Medicare coverage for patients with end-stage renal disease (ESRD). The National Organ Transplant Act of 1984 initiated the process of creating what later became UNOS, an umbrella organization to ensure access to organs by patients in need, to enhance organ procurement and allocation, and to improve posttransplant outcomes. This infrastructure later became the blueprint for other countries to follow. As a result, organ transplantation is the most transparent field of medicine. Data such as transplant center performance are readily available on public websites; penalties for violation of regulations and for underperformance often result in transplant programs being shut down.
Today, a kidney transplant remains the most definitive and durable renal replacement therapy for patients with ESRD. It offers better survival and improved quality of life and is considerably more cost-effective than dialysis.69,70 According to the 2010 Scientific Registry of Transplant Recipients (SRTR) annual report, a total of 84,614 adult patients were on the kidney transplant waiting list, including 33,215 added just that year.71 Yet in 2009, only 15,964 adult kidney transplants were performed in the United States (9912 with a deceased donor and 6052 with a living donor). Of note, the number of patients added to the kidney transplant waiting list has increased every year, but the number of kidney transplants performed has been declining since 2006. On the positive side, posttransplant outcomes have continued to improve: in 2009, the 1-year graft survival rate with a living donor kidney was 96.5%; with a deceased donor kidney, the rate was 92.0%.
The advantages of a living donor kidney transplant include better posttransplant outcomes, avoidance of prolonged waiting time and dialysis, and the ability to coordinate the donor and recipient procedures in a timely fashion. Living donor kidney recipients enjoy better long-term outcomes, a low incidence of delayed graft function, and reduced risks of posttransplant complications. Furthermore, the elective nature of living donor kidney transplants provides unique opportunities for recipient desensitization treatment if the donor and recipient are ABO- incompatible or if the HLA cross-match results are positive.
Some of the challenges transplant professionals face today are closing the growing gap between supply and demand and thereby reducing the current prolonged waiting times; refining immunosuppressive medications to achieve better outcomes with reduced toxicity; and caring for patients who develop rejection, especially antibody-mediated rejection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree