Total Mesorectal Excision with Coloanal Anastomosis: Laparoscopic Technique
John H Marks
Elsa B. Valsdottir
DEFINITION
Total mesorectal excision with coloanal anastomosis via a transanal abdominal transanal proctosigmoidectomy (TATA) is defined as the complete removal of the embryologic tissue block of the rectum, leaving the sphincter muscles intact and thus avoiding a permanent stoma. Neoadjuvant chemoradiotherapy is an essential component to successful sphincter preservation. The abdominal part of the procedure can be performed with laparoscopic technique.
DIFFERENTIAL DIAGNOSIS
Several conditions, both benign and malignant, can have similar presentation to rectal cancer. These include adenomatous polyps, solitary rectal ulcer, radiation injury, carcinoid tumor, and squamous cell carcinoma. Hence, a tissue biopsy confirming the diagnosis of rectal cancer is imperative.
PATIENT HISTORY AND PHYSICAL FINDINGS
Careful patient selection is crucial for successful sphincter preservation in rectal cancer. A detailed history and physical examination are mandatory. Contraindications include inability to receive neoadjuvant chemoradiation therapy for distal rectal cancers, either because of comorbidities or previous radiation to the pelvis, previous radical surgery on rectum, distance of tumor from the dentate line, invasion of tumor into the sphincter muscles after completion of neoadjuvant chemoradiation therapy, and fecal incontinence on presentation.
Detailed past medical history is obtained, emphasizing fecal continence, bowel habits, personal and family history of cancers and current medications and allergies, previous radiation to the pelvis, and previous abdominal surgeries.
Prior radiation therapy for other cancers in the pelvis, such as cervical or prostate, is usually a contraindication. It is, however, helpful to review the previous records with regard to the dose and field treated and make decisions on individual basis.
A detailed family history of cancers can help identify increased risk for other types of cancer as well as identify family members who are at an increased colon cancer risk. Recommendations should be given to first-degree relatives with regard to screening.
Patient age, nodal status, or tumor size are not contraindications for sphincter preservation as long as the patient is a reasonable surgical candidate and negative margins (distally and circumferentially) can be obtained.
Physical examination must include a thorough abdominal exam, including palpation of inguinal lymph nodes bilaterally. Most importantly, a careful digital rectal exam and a rectoscopy or flexible sigmoidoscopy are performed.
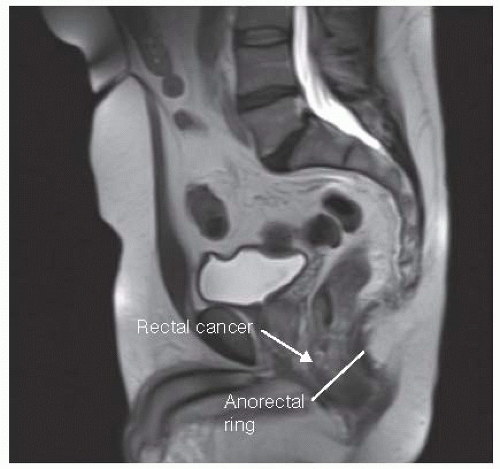
The location of the tumor (anterior, posterior, or lateral), distance from the anorectal ring, size, fixity, circumference, configuration, and ulceration of the tumor need to be documented. This is imperative in preoperative planning as well as allowing for assessment of response to neoadjuvant treatment later (FIG 1).
IMAGING AND OTHER DIAGNOSTIC STUDIES
All patients require preoperative staging of the disease with regard to the tumor, lymph node status, and distant spread, both clinically and radiographically.
The most accurate way to determine size, length, and depth of the tumor invasion as well as any enlarged lymph nodes is with magnetic resonance imaging (MRI) or endoscopic rectal ultrasound (ERUS) (FIG 2).
A computed tomography (CT) of the abdomen and chest should be obtained to evaluate for distant spread of the disease to the liver or lungs.
Preoperative blood work should include a hemogram, blood chemistries, and a carcinoembryonic antigen (CEA) level.
Full colonoscopy is needed to evaluate the entire colon for other pathology and synchronous malignant lesions or polyps.
Histologic assessment with biopsy of the primary tumor is necessary and usually obtained at the time of colonoscopy.
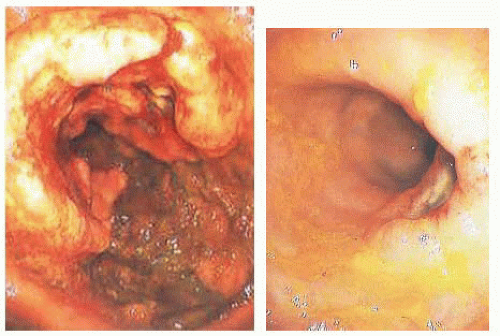 FIG 1 • Rectal cancer before (left) and after (right) neoadjuvant chemoradiation. This patient had a good response to treatment. |
SURGICAL MANAGEMENT
Preoperative Planning
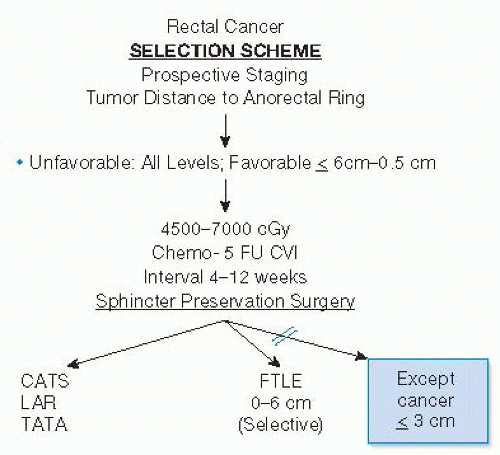
Neoadjuvant chemoradiotherapy is the key to successful sphincter preservation. The radiation therapy is a high-dose, long-term treatment to maximize tumor downstaging. Preferred radiation dose is 5,580 cGy, with 4,500 cGy to the entire pelvis with a boost to the presacral area and tumor location, delivered over the course of 5 weeks. Concurrent chemotherapy based on 5-fluorouracil (5-FU) either orally or intravenously increases the sensitivity of the tissues to radiation, enhancing efficacy (FIG 3).
Neoadjuvant chemoradiation apoptotic effect occurs only at cell division. Maximum cytotoxic effect is 8 to 12 weeks after completion of treatment. Extending the interval between completion of radiation therapy and surgery up to 8 to 12 weeks therefore gives the patient the fullest benefit of the treatment, maximizing downstaging and extending the options for sphincter preservation.
Decision regarding sphincter preservation should be based on the status of the cancer after completion of chemoradiotherapy. All patients, except those whose cancers remain fixed at or below the 3-cm level are offered sphincter preservation.
Accepting a distal margin of resection from the cancer as small as 5 mm is necessary for very low tumors. This does not adversely affect outcome. Dissection is started transanally to assure a known distal margin. This is particularly helpful in the postirradiated rectal cancer where there is often only a small scar left, making decisions as to where a stapler would be placed from above difficult.
The patient should receive a bowel preparation the day before surgery.
Perioperative antibiotics and deep vein thrombosis prophylaxis should be given.
Positioning
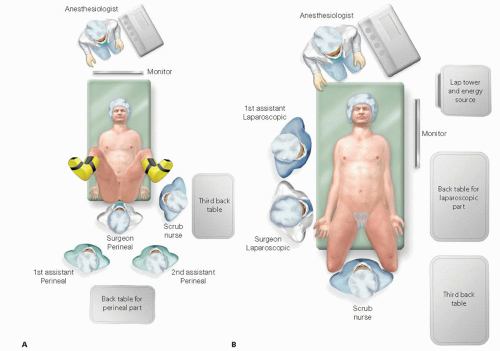
The operation has both an abdominal part and perineal part. The surgeon and first assistant stand between the legs during the perineal part. For the abdominal part, which is performed laparoscopically, they stand at the patient’s right side. It is important that the surgical team is free to move around the patient. The laparoscopic equipment and energy sources are positioned to patient’s left (FIG 4).
The patient is placed in the lithotomy position with the buttocks extending 2 cm over the padded table edge. Both arms are padded and tucked. The chest is taped to the table to further prevent slipping of the patient as the table is maneuvered. The Foley catheter is taped over the right thigh. The abdomen is prepped with Betadine and the perineum with povidone-iodine. In women, the vagina is prepped with povidone-iodine.
TECHNIQUES
TRANSANAL, INTERSPHINCTERIC RESECTION OF RECTUM
Place a sponge soaked in povidone-iodine in the anal canal or irrigate it with povidone-iodine. In order to minimize the possibility of dislodging tumor cells, avoid digitalizing the canal after this.
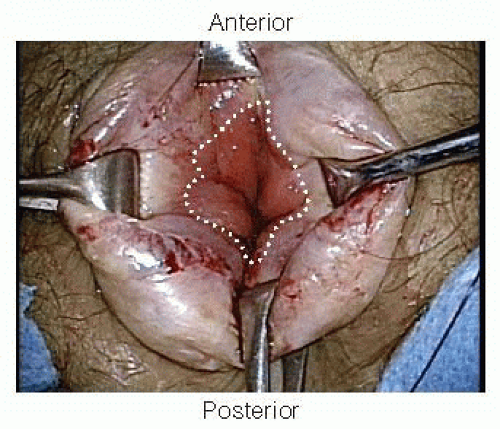
To allow visualization of the dentate line, Alice-Adair clamps are placed circumferentially around the anal canal to evert the anal tissue (FIG 5).
The dentate line is incised circumferentially with electrocautery through the mucosa, thus defining the distal resection margin. This is a critical step to avoid radial tearing later in the dissection (FIG 6).
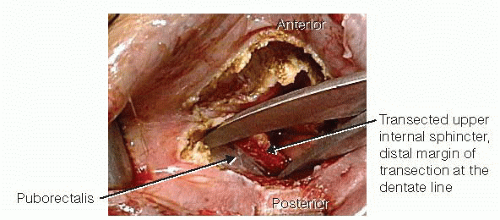
The Metzenbaum scissors are spread posterolaterally and slightly off the midline, perpendicular to the axis of the anus, to enter into the plane between the transected upper half of the internal sphincter and the underlying puborectalis. This plane is developed circumferentially (FIG 7).
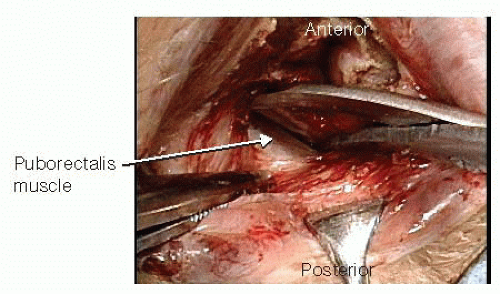
Alice-Adair clamps are applied to the transected distal portion of the rectum to facilitate retraction. One never applies more than four clamps at a time, as this is usually too bulky. The shiny, glistening white aspect of the puborectalis is identified using the scissors. Visualization of this white tissue is the key to ensuring that the dissection is carried out in the proper plane (FIG 8). Placing a small Deaver retractor allows development of the plane between the rectum and the levator ani complex. Once the proper plane is entered, the dissection is essentially bloodless.
The sharp dissection is brought around anteriorly (FIG 9). In women, a finger in the vagina allows palpation of the vaginal wall, and it is generally not a problem to avoid this structure. In men, one has to be careful when proceeding anteriorly to avoid taking the dissection anterior to the prostate. The length of dissection cephalad is up to the seminal vesicles in men and to the cervix in women. This dissection is carried circumferentially until the rectum is fully mobilized (FIG 10).
The rectum is oversewn in a watertight fashion with a 0-Vicryl stitch, turning the edges inward to avoid potential spilling of feces or tumor cells during the abdominal part of the procedure. The pelvis is irrigated from below with saline; a sponge is placed through the anus with an occlusive dressing in the perineum.
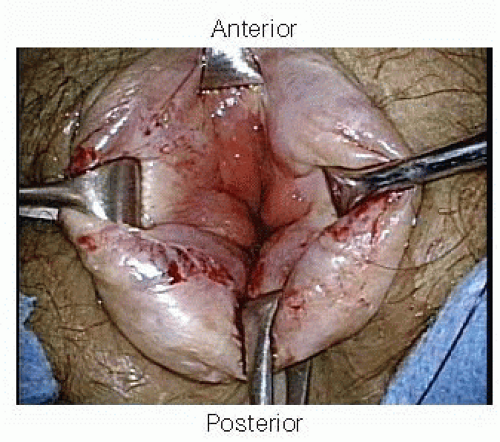 FIG 5 • To allow visualization of the dentate line, Alice-Adair clamps are placed circumferentially around the anal canal to evert the anal tissue. |
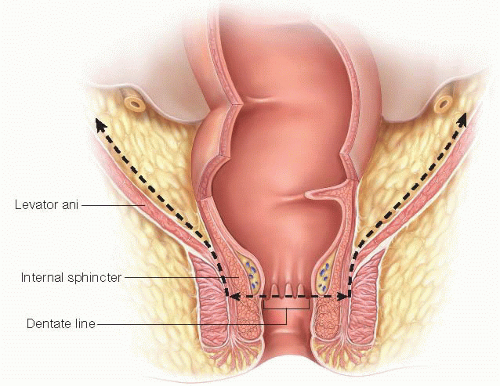 FIG 9 • The drawing shows the lines of pelvic dissection.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|