Thymoma
Key Facts
Clinical Issues
Majority of thymomas are very low-grade malignant neoplasms with generally indolent behavior
Status of capsular integrity is most important determinant factor for prognosis
Microscopic Pathology
WHO classification system is based predominantly on cell type, cytologic atypia, and proportion of lymphocytes to epithelial cells: Thymoma type A, AB, B1, B2, B3, and thymic carcinoma
2 basic cell types are recognized: Spindle/oval and round/epithelioid
Type A thymoma: Composed of oval or spindle cells with scattered nuclear chromatin and inconspicuous or absent nucleoli without mitotic activity
Type AB thymoma: Composed of oval or spindle cells identical to those seen in type “A” but admixed with abundant small lymphocytes
Type B1 thymoma: Composed of round/epithelioid cells with single small eosinophilic nucleoli and abundant cytoplasm, admixed with numerous small T-lymphocytes
Type B2 thymoma: Composed of approximately equal admixture of round epithelial cells and small lymphocytes
Type B3 thymoma: Composed of sheets of large epithelioid cells admixed with scant lymphocytes
Thymic carcinoma (type C thymoma): Characterized by overt cytologic features of malignancy and absence of organotypical thymic features; resembles carcinomas at other sites
TERMINOLOGY
Synonyms
Primary thymic epithelial neoplasm
Definitions
Primary thymic epithelial neoplasm composed of thymic epithelial cells admixed in varying proportions with immature T-lymphocytes
ETIOLOGY/PATHOGENESIS
Pathogenesis
Unknown
Close association with myasthenia gravis and other autoimmune disorders
CLINICAL ISSUES
Presentation
Chest pain
Shortness of breath
Paraneoplastic syndrome (myasthenia gravis, hypogammaglobulinemia, pure red cell aplasia, etc.)
Superior vena cava syndrome
Asymptomatic in up to 30% of cases
Incidental finding on routine chest x-ray or during coronary artery bypass surgery
Natural History
Majority of thymomas are very low-grade malignant neoplasms with generally indolent behavior
Size and status of capsular integrity are 2 important determinant factors for prognosis
Invasive tumors are associated with more aggressive behavior
Incompletely excised tumors have tendency to recur locally and spread along chest cavity
Recurrences can take place many years after initial resection (i.e., > 10-15 years)
Most common sites for metastases are lung, pleura, and thoracic lymph nodes
Extrathoracic metastases are extremely rare (< 2% of cases)
Treatment
Complete surgical excision for noninvasive tumors
Radiation therapy for incompletely resected tumors
Surgical excision + postoperative radiation therapy for invasive tumors
Repeat surgical excision + radiation for recurrent tumors
Combination chemotherapy for advanced stage and metastatic tumors
Best chance for cure is complete surgical excision with negative margins
Prognosis
Most important prognostic factor is clinical staging (Koga modified Masaoka scheme)
80-90% survival at 15 years with stages I and II
70% survival at 15 years with stage III
60% survival at 5 years with stage IV
Stages I and II include infiltration of capsule and minimal invasion of perithymic fat (collectively regarded as “noninvasive” tumors; confined to anterior mediastinum)
Stages III and IV include infiltration of adjacent or neighboring structures, implants, and distant metastases (collectively regarded as “invasive”)
IMAGE FINDINGS
General Features
Radiographic Findings
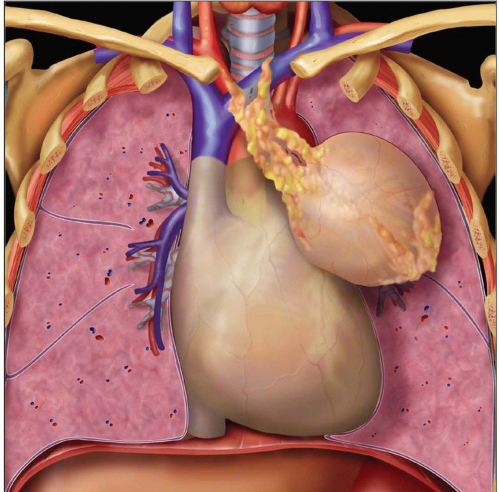
Round or oval anterior mediastinal mass
Usually centered over heart; best seen on lateral view
Linear and peripheral calcifications in capsule (10% of patients)
MR Findings
T1WI: Isointense relative to muscle
T2WI: Hyperintense, approaching that of fat
CT Findings
CECT best imaging tool for thymoma
Oval or lobulated mass within anterior mediastinum
Homogeneous enhancement is common in small tumors
Heterogeneous enhancement more common in large tumors
Thin and linear calcifications seen within capsule in 1/3 of patients
Cystic changes and necrosis common in larger tumors
Obliteration of mediastinal fat planes or mediastinal structures seen in invasive tumors
MACROSCOPIC FEATURES
General Features
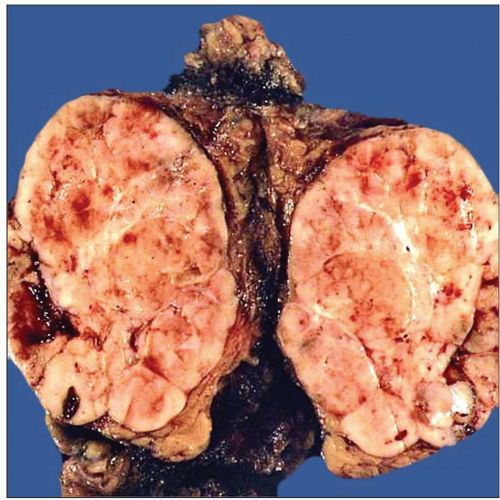
Generally well-circumscribed, encapsulated, solid tumor
Homogeneous tan-white, rubbery tissue on cut surface
Lobulated cut surface
May be cystic and multilocular
May contain calcifications in capsule or within tumor
Rarely can be multifocal
Can be ectopically located in posterior mediastinum, lung, neck, or pleura
Can show areas of necrosis and hemorrhage
Invasive tumors usually compromise adjacent structures, including large vessels, pericardium, pleura
Distant metastases are rare
Sections to Be Submitted
At least 1 section per cm of greatest tumor diameter
Take additional sections if tumor shows variegated appearance
Sample solid areas in cyst walls in multicystic tumors
Always include sections of inked outer surface of specimen
Coordination with surgeon should be sought to establish “true” margins to be sampled
Any structures attached to specimen (i.e., pleura, lung, large vessels) should be inked and sampled separately as they represent “true” margins
MICROSCOPIC PATHOLOGY
Histologic Features
Histologic classification is controversial
Currently 2 systems are in use: Suster & Moran classification and WHO classification
Suster & Moran classification: Based on degree of organotypical differentiation, divided into 3-tiered system
Well-differentiated (thymoma)
Moderately differentiated (atypical thymoma)
Poorly differentiated (thymic carcinoma)
WHO classification is based primarily on cell type, cytologic atypia, and proportion of lymphocytes to epithelial cells
WHO type A: Composed of spindle cells without cytologic atypia
WHO type AB: Composed of spindle cells admixed with abundant lymphocytes
WHO type B1: Composed of round, epithelioid cells admixed with abundant lymphocytes
WHO type B2: Composed of round, epithelioid cells admixed with equal amounts of lymphocytes
WHO type B3: Composed predominantly of epithelial cells with cytologic atypia
WHO classification has a series of other distinctive histologic types that do not fit into the standard categories
“Metaplastic” thymoma
Multifocal thymoma
Microscopic thymoma
Micronodular thymoma
Anaplastic thymoma
Older (“traditional”) classification by Bernatz et al from Mayo Clinic is still used today by many and divides these tumors based on their cell composition
Lymphocyte-rich thymoma
Mixed, lymphoepithelial thymoma
Epithelial-rich thymoma
Spindle cell thymoma
Cytologic Features
2 basic cell types are recognized
Oval/spindle cells (types A, AB)
Round/epithelioid cells (types B1-3)
Type A thymoma is composed of oval or spindle cells with scattered nuclear chromatin and inconspicuous or absent nucleoli and no mitotic activity
Spindle cell thymoma usually contains few lymphocytes
Majority of spindle cell thymomas are low grade and encapsulated
Invasive or atypical spindle cell thymoma can follow aggressive behavior
Distant metastases and death can occur in some cases of spindle cell thymoma
Type AB thymoma is composed of oval or spindle cells identical to those in type “A” but admixed with abundant small lymphocytes.
Spindle cells do not display mitotic activity
Lymphocytes admixed with epithelial cells are of T-cell type
Tumors usually contain admixture of lymphocyte-rich and lymphocyte-poor areas
Tumors may be composed exclusively of lymphocyte-rich areas and be confused for B1 thymoma
Type B1 thymoma is composed of round/epithelioid cells with single small eosinophilic nucleoli and abundant cytoplasm, admixed with numerous small T-lymphocytes
Small lymphocytes predominate and overshadow epithelial cells
Contains frequent perivascular spaces and areas of “medullary” differentiation
Equivalent to “lymphocyte-rich” or “lymphocytepredominant” in traditional classification (Mayo Clinic)
Type B2 thymoma is composed of approximately equal admixture of round epithelial cells and small lymphocytes
Epithelial cells may show mild degree of atypia and enlargement of nuclei
Admixtures with B1 areas may be seen in about 30% of cases
Equivalent to “mixed lymphoepithelial” thymoma of traditional classification (Mayo Clinic)
Type B3 thymoma is composed of sheets of large epithelioid cells admixed with scant lymphocytes
Epithelial cells are characterized by nuclear enlargement with dense chromatin pattern and prominent nucleoli
Mitotic figures can be encountered in epithelial cells
Cell nuclei show tendency to adopt raisin-like configuration
Cytoplasm of tumor cells is usually abundant, eosinophilic, and with sharp cell borders
Tendency for epithelial cells to palisade around perivascular spaces
Tumor cells can also be oval or spindle with similar nuclear features
Type A thymomas may exhibit unusual growth patterns
Hemangiopericytic growth pattern
Micronodular growth pattern with lymphoid B-cell hyperplasia
Biphasic pattern with pseudosarcomatous stroma (“metaplastic” thymoma)
Skin adnexal-like (“adenoid”) growth pattern
Sclerosing growth pattern
Rosette-forming growth pattern
Type B thymomas may exhibit unusual features
Extensive multilocular thymic cyst-like changes
Areas of infarction, hemorrhage, and necrosis
Massive infiltration by plasma cells in stroma
Clear cell changes
“Starry sky” appearance simulating lymphoma
Type C thymoma (thymic carcinoma) is characterized by overt cytologic evidence of malignancy and absence of organotypical features of thymic differentiation
Diagnosis of exclusion
Requires demonstration of absence of tumor elsewhere clinically and radiographically
Lymphatic/Vascular Invasion
Very rare; unknown significance but generally associated with worse prognosis
Margins
Very difficult to determine without assistance of surgeon
True resection margins need to be inked or tagged by surgeon before submitting to pathology
Inked anterior surface does not represent “margin” unless it was invading at time of surgery
True margins need to be inked for proper assessment
Lymph Nodes
Thymoma rarely metastasizes to lymph nodes
Majority of lymph node metastases in thymoma are to mediastinal nodes
Other intrathoracic lymph nodes may also be involved more rarely by metastatic thymoma
DIFFERENTIAL DIAGNOSIS
Lymphoblastic Lymphoma
Does not show scattered keratin-positive cells admixed with the immature T-lymphocytes
Shows rapid growth with sudden onset of symptoms
Thymoma is slow-growing tumor with slowly progressive symptoms
Most common age for lymphoblastic lymphoma is childhood and adolescence
Most common age for thymoma is in middle-aged adults; rare in children and adolescents
Acquired Multilocular Thymic Cyst
Multilocular cysts do not contain discrete areas attached to walls of cysts showing typical type B thymoma
Type A thymoma can undergo massive cystic changes, but the cells lining the cysts are spindle cells
Shows small cuboidal or squamous epithelial cells lining the cysts in continuity with dilated Hassall corpuscles
Shows prominent lymphoid follicular hyperplasia and severe acute and chronic inflammation with cholesterol cleft granulomas
Hemangiopericytoma/Solitary Fibrous Tumor
Spindle cells in solitary fibrous tumors are not keratin-positive
Spindle cells in solitary fibrous tumor are CD34, Bcl-2, and CD99 positive
Shows characteristic linear pattern of stromal collagenization resulting in deposition of rope-like collagen separating spindle cells
Devoid of immature T cells
Spindle cell thymoma may contain variable number of immature T cells admixed with epithelial cells
Neuroendocrine Carcinomas
Positive for neuroendocrine markers
Rosette-like structures in thymoma are only positive for cytokeratin
Thymic carcinoids usually show increased mitotic activity and tumor cells necrosis
Tumor cells in carcinoids show characteristic stippled (“salt and pepper”) chromatin pattern
Other features of thymic carcinoids include a nested growth pattern (“zellballen”) and formation of trabeculae, ribbons, and festoons
DIAGNOSTIC CHECKLIST
Clinically Relevant Pathologic Features
Encapsulation/circumscription
Presence or absence of cytologic atypia in epithelial cells
Size of tumor
Clinical stage
Presence or absence of myasthenia gravis
Pathologic Interpretation Pearls
Great variation in histologic appearance due to tumor heterogeneity
Identification of scattered keratin-positive neoplastic cells in lymphocyte-rich tumors
Hemorrhage and necrosis may be seen in encapsulated low-grade, well-differentiated tumors; not to be mistaken for ominous sign
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree