Thoracic Aortic Stent Graft Repair for Aneurysm, Dissection, and Traumatic Transection
Brant W. Ullery
Jason T. Lee
DEFINITION
In 1994, Dake and colleagues,1 at Stanford University, were the first to report the use of custom-designed thoracic aortic stent grafts for the treatment of descending thoracic aortic aneurysms in patients deemed high risk for conventional open surgery. Each of these devices was deployed through peripheral arterial access, successfully excluding the aneurysm from systemic pressurization. This groundbreaking minimally invasive technique thereby avoided many of the physiologic insults associated with open surgery, including the need for thoracotomy, aortic cross-clamping, reperfusion injury, and acute hemodynamic changes.
Results from the first multicenter U.S. Food and Drug Administration-sponsored trial for thoracic aortic stent grafts demonstrated significantly less perioperative mortality, respiratory failure, renal insufficiency, and spinal cord ischemia in patients after thoracic endovascular aortic repair (TEVAR) compared to a matched cohort of patients undergoing open descending thoracic aortic aneurysm repair.2
After two decades of surgeon experience and endovascular technologic advancement, TEVAR has evolved to serve as a primary treatment strategy for an increasingly diverse group of acute and chronic aortic pathologies including thoracic aortic aneurysms, dissections, and traumatic transections.
DIFFERENTIAL DIAGNOSIS
Depending on the type and extent of pathology, TEVAR may include the use of fenestrated or branched stent grafts, advanced snorkel/chimney/periscope techniques, or the need for hybrid debranching procedures. The decision to treat thoracic aortic pathology with stent grafts is based on individual patient comorbidity burden, detailed analysis of thoracic aortic anatomy, and physician experience.
Acute thoracic aortic pathologies often present with chest pain and therefore must be considered in the workup for acute coronary syndrome. The ubiquitous use of computed tomography (CT) scanning for pain, shortness of breath, trauma, and to “rule out” many pathologies has led to an increase in the recognition of thoracic aortic pathology potentially benefitting from TEVAR technology.
PATIENT HISTORY AND PHYSICAL FINDINGS
Thoracic aortic aneurysms (TAAs) are defined as localized or diffuse dilation of 50% or more relative to the diameter of the adjacent normal-sized aorta. Common risk factors for aneurysmal degeneration include smoking, hypertension, chronic obstructive pulmonary disease, atherosclerosis, and connective tissue diseases. Indications for repair of descending TAAs are similar to those for conventional open repair: maximum aortic diameter greater than 6 cm, rapid aneurysmal growth (>5 mm of growth over 6 months), or symptoms such as persistent chest or back pain, rupture, or dissection. In most patients with TAA, the aneurysms were diagnosed following routine imaging ordered for other reasons and are therefore most commonly asymptomatic.
Aortic dissection occurs when an intimal tear in the aorta causes blood to flow between the layers of the wall of the aorta and most often presents as tearing chest pain that radiates to the back. Potential etiologic factors leading to aortic dissection include poorly controlled hypertension, connective tissue disorders, trauma, or vasculitis. Medical management of uncomplicated type B thoracic aortic dissection serves as the current standard of care. These practice guidelines stem from the results of the INvestigation of STEnt grafts in patients with type B Aortic Dissection (INSTEAD) trial, the first prospective, multicenter randomized trial comparing optimal medical therapy (e.g., blood pressure control) to TEVAR for uncomplicated type B dissection.3 This trial demonstrated no significant improvement in 2-year survival or adverse event rates with TEVAR despite favorable aortic remodeling, although recently reported 5-year data suggest improved long-term survival in patients undergoing TEVAR. In contrast, for patients with complicated type B dissections involving rupture, malperfusion (e.g., visceral or limb ischemia), or refractory back pain despite optimal medical management, TEVAR is indicated. The goal of TEVAR in this setting is to cover, or exclude, the primary entry tear and reexpand the true lumen while promoting thrombosis of the false lumen.
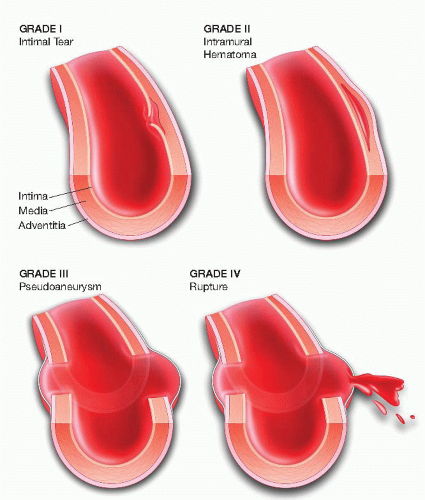
Traumatic aortic transection results from a high-velocity or deceleration injury to the aorta. The tethering of the aorta by the ligamentum arteriosum makes this site most susceptible to shearing forces during sudden deceleration. A high index of suspicion is necessary to help make the diagnosis. Trauma workups most often involve whole-body CT scanning, which allows rapid triage for possible treatment. CT-A commonly demonstrates an irregular outpouching beyond the takeoff of the left subclavian artery at the aortic isthmus, which corresponds to the presence of an aortic pseudoaneurysm caused by the traumatic event. Extent of blunt traumatic aortic injury and the corresponding physiologic insult may range from clinically occult intimal injury to life-threatening complete transection and rupture (FIG 1).4 Early diagnosis and endovascular treatment is generally recommended for those presenting with a traumatic aortic transection, particularly when there is a contour abnormality visualized on cross-sectional imaging.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Transesophageal echocardiography (TEE) may serve as a useful imaging tool, particularly in the setting of acute thoracic aortic pathology. TEE can confirm the presence of aortic dissection, distinguish between types A and B dissections, identify involvement of supra-aortic vessels, and assess for contained rupture.
High-resolution computed tomography angiography (CT-A) with three-dimensional reconstructive software allows for the most complete anatomic analysis, including details regarding aneurysm morphology, diameter, dissection flap characterization, thrombus burden, calcification, angulation, and branch vessel orientation.
Familiarity and routine usage of three-dimensional workstations and the ability to customize measurements provide an accurate road map to guide endovascular strategy, device selection, and stent graft sizing.
SURGICAL MANAGEMENT
Preoperative Planning
Patients scheduled for elective TEVAR undergo routine preoperative cardiac evaluation. Based on cardiovascular risk profile, symptomatology, and presence of electrocardiogram abnormalities, selected patients undergo further evaluation in the form of an exercise stress test, dobutamine stress echocardiography, or Persantine thallium stress testing. Coronary angiography is pursued in cases involving extensive or symptomatic coronary artery disease.
Aortic transections or symptomatic dissections and aneurysms should have early and aggressive blood pressure control using intravenous beta-blocker or calcium channel blocker medications. After obtaining a reliable clinical examination, refractory chest, back, or abdominal pain should be treated with narcotic analgesics.
Renal protective strategies should be employed preoperatively to minimize the risk of contrast-induced nephropathy. Intravenous hydration is initiated preoperatively and, in the setting of baseline renal insufficiency, may warrant early hospital preadmission and concomitant administration of Mucomyst and bicarbonate infusion.
Suspected blunt aortic injury should prompt a referral to a level I trauma center in order to facilitate early evaluation by a vascular specialist and other pertinent members of a multidisciplinary trauma team.
General anesthesia is routinely performed in TEVAR cases. Prophylactic lumbar cerebrospinal fluid (CSF) drainage is
considered in every case based on the relative risk of spinal cord ischemia, hemodynamic status, and acuity of clinical presentation. Arterial monitoring is performed via a right radial artery approach. Peripheral intravenous lines are typically adequate; however, more intensive central venous monitoring may be required in cases involving unstable traumatic transections, patients with significant baseline cardiovascular comorbidities, or any case involving hemodynamic instability.
Preoperative imaging should be heavily scrutinized for the adequacy of iliofemoral access anatomy. An iliac conduit may be required in cases involving small-caliber, tortuous, or heavily calcified access vessels. Anticipated use of a conduit should prompt consideration of an autotransfusion or cell saver machine to be available during the procedure.
Numerous variables have been identified as risk factors for the development of spinal cord ischemia after TEVAR. Given that hypoperfusion represents the primary etiology of spinal cord injury following TEVAR, commonly cited risk factors involve those relating to the extent of impairment or exclusion of the collateral perfusion to the spinal cord. The European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) investigators reported results from the largest multicenter registry to date (N = 606).5 In the EUROSTAR registry, the incidence of spinal cord ischemia was 2.5% and independent risk factors included left subclavian artery coverage without revascularization (odds ratio [OR], 3.9; p = .037), concomitant open abdominal aortic surgery (OR, 5.5; p = .037), and the use of three or more stent grafts (OR, 3.5; p = .043).
Based on the principle that spinal cord perfusion pressure is approximated by the difference between the mean arterial pressure (MAP) and CSF pressure, placement of a prophylactic lumbar drain has the potential to increase spinal cord perfusion pressure by decreasing CSF pressure and may be beneficial in select patients at high risk for spinal cord ischemia. Percutaneous drainage of CSF is performed by inserting a silastic catheter 10 to 15 cm into the subarachnoid space through a 14-gauge Tuohy needle at the L3-L4 vertebral interspace. The open end of the catheter is attached to a sterile closed circuit reservoir and the lumbar CSF pressure is measured with a pressure transducer zero-referenced to the midline of the brain. Lumbar CSF can be drained continuously or intermittently in the operating room to achieve target CSF pressures of 10 to 12 mmHg. Postoperatively, intermittent or continuous CSF drainage can be continued in the intensive care unit for CSF pressures exceeding 10 mmHg or at the first sign of lower extremity weakness. In the absence of neurologic deficits, the lumbar CSF drainage catheter can be clamped 24 hours postprocedure followed by continued monitoring of CSF pressure together with serial neurologic assessments. The CSF drain can then be removed at 48 hours after operation. Although prophylactic or therapeutic lumbar CSF drainage has an established record of safety, complications have been reported to occur in approximately 1% of patients, which may include neuraxial hematoma, subdural hematoma, catheter fracture, meningitis, intracranial hypotension, chronic CSF leak, and spinal headache.
Selection and Sizing of Thoracic Stent Graft
Landing zones
Proximal and distal landing zones must be of sufficient length (usually at least 2 cm) to enable safe and accurate deployment bracketing the area of thoracic aortic pathology, which often includes the subclavian artery proximally or the celiac artery distally.
Intentional coverage of the left subclavian artery is sometimes required due to a very proximal extent of aortic pathology, especially transections. Left subclavian artery revascularization may be required in select cases. The celiac artery rarely requires intentional coverage.
Significant tortuosity, circumferential mural thrombus, and extensive calcification can compromise the proximal or distal landing zone, thereby predisposing to inadequate fixation and subsequent development of endoleak or migration. Site of proximal and distal landing zones should be selected in order to minimize the impact of these anatomic features, even if it requires extending the length of aortic coverage.
A variety of anatomic measurements are taken from preoperative CT-A imaging to assist in the sizing and selection of the thoracic stent graft (FIG 2). Interventionalists should be proficient in accurate sizing and measuring of key thoracic aortic locations that influence device selection and ultimately determine patient outcomes.
Sizing of stent grafts
The degree of stent graft oversizing can vary based on the indication for intervention. Stent grafts are generally oversized by 10% to 20% based on the aortic diameter at the proximal and distal fixation sites for aneurysmal disease. Insufficient oversizing for the treatment of TAAs may predispose to inadequate exclusion and the potential for endoleak or migration. Aggressive oversizing, on the other hand, increases the risk for stent graft collapse, graft thrombosis, access arterial injury, and potential for peri- or postprocedural iatrogenic retrograde type A dissection.
Chronic type B dissections are frequently characterized by a thick, nonmobile dissection flap, or septum, that separates true and false lumens into concave or convex discs of flow lumen. Such dissection flaps have limited compliance; therefore, minimal or no oversizing may be required in order to achieve a suitable proximal or distal seal.
Aortic transections frequently occur in young trauma patients with normal or minimally diseased aortas. As such, minimal oversizing is needed to achieve an adequate seal and only recently did device manufacturers create devices meant for smaller diameter aortas. Note also that underrescucitated patients on admission will have smaller aortic diameters on their CT-A.
Currently available stent grafts range in diameter from 22 to 46 mm. Given the traditional 10% to 20% rule of device oversizing, these devices are designed to safely treat aortas with landing zones ranging from 19 to 43 mm in diameter.
Access vessel anatomy
Current thoracic aortic stent grafts require large-caliber delivery systems, ranging from 18 to 26 Fr in outer diameter. Small, tortuous, and heavily calcified iliofemoral arteries may prohibit sheath advancement and predispose to access site-related complications, including groin hematoma, dissection, or rupture.
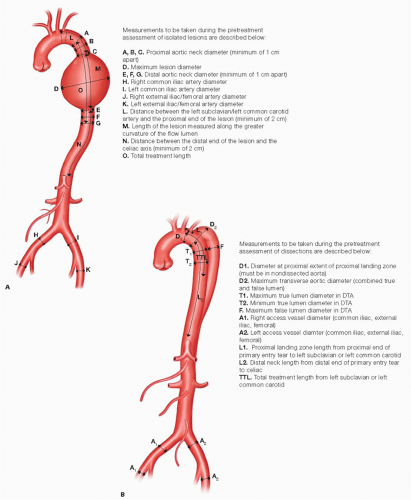
FIG 2 • Anatomic measurements to assist in thoracic stent graft device sizing and selection for the treatment of aneurysms (A) and dissections (B). DTA, descending thoracic aorta.
Careful evaluation of access vessel anatomy on preoperative imaging should be performed in order to assess the caliber, tortuosity, thrombus burden, and extent of calcification of the iliofemoral arteries. Such anatomic information will serve as the basis for deciding laterality of femoral access as well as to determine the need for an iliac conduit.
Serial dilation may be attempted for patients with small iliofemoral vessels. Iliac atherosclerotic lesions may be pretreated with balloon angioplasty and/or stent grafting in order to facilitate sheath advancement and introduction of the thoracic stent graft components.
Iliac conduits serve as a safe and reliable technique to circumvent issues related to suboptimal access vessel anatomy. From either flank incision, a retroperitoneal exposure provides visualization of the common iliac artery or distal abdominal aorta. A 10- or 12-mm Dacron graft is commonly used as the conduit of choice. The conduit can be modified by creating a patch at the distal end in order to further facilitate the delivery of largecaliber sheath and enable additional degrees of torqueability (FIG 3). This modification involves creating a patch by cutting the Dacron graft along its long access, thereby enlarging the transition zone from the graft to artery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree