Noninvasive procedures
Seizurogenic
Electroconvulsive therapy (ECT)
Magnetic seizure therapy (MST)
Focal electrically administered seizure therapy (FEAST)
Nonseizurogenic
Transcranial magnetic stimulation (TMS)
Transcranial direct current stimulation (tDCS)
Invasive procedures
Nonseizurogenic
Vagus nerve stimulation (VNS)
Deep brain stimulation (DBS)
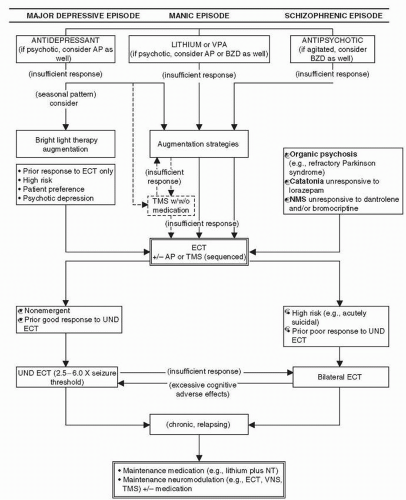 Figure 8-1 The role of therapeutic neuromodulation in the treatment of psychiatric disorders. (Adapted from Dowd SM, Janicak PG. How effective and safe is rTMS? Current Psychiatry. 2003;2:59-66.) |
(18,19,20 and 21). In this context, Kramer (21) and the American Psychiatric Association (APA) Task Force on ECT (22) have presented outlines for teaching ECT to address deficits in training programs.
Neurotransmitters effects
Neuroendocrine effects
Neurophysiological effects
Neurotrophic effects
The use of brain tissue in animals versus peripheral tissues in humans, as well as the differential effects of ECS in animals versus ECT in humans, respectively
A predominance of NE β1-receptors in the CNS and of NE β2-receptors in peripheral tissues
Findings of receptor activity differences in normal young animals, with their own speciesspecific biochemistry and physiology, which cannot be easily generalized to baseline and posttreatment differences in normal humans or depressed patients
at the receptor level to a variety of intracellular effectors. Abnormalities in G-protein expression or function are implicated in various medical and neuropsychiatric disorders, and several psychotropics are known to affect G-protein activity (36). Given their ubiquitous role in neuronal function, it is plausible that G-proteins are involved in psychiatric disorders (e.g., major depression). Existing treatments and potential novel, site-specific drugs that target these proteins may help clarify pathophysiology, as well as the mechanism of action of various drug and neuromodulation therapies. In this context, studies have shown that G-proteins (inhibitory and stimulatory) in mononuclear leukocytes and platelets are decreased in depression and increased in bipolar disorder (37,38 and 39). Further, ECT has been shown to normalize G-protein function in both conditions (40). This normalization precedes and predicts improvement in depressed patients receiving ECT. One possibility is that ECT may stabilize dysregulated intracellular signaling, which explains why it is effective for both mania and depression. Of note, lithium and antidepressants can also modulate Gprotein functions (41,42) (see Chapters 7 and 10).
Increasing the seizure threshold (ST)
Decreasing the overall duration of an episode
Decreasing neurometabolic response to an episode
Decreasing the phenomenon of amygdaloid kindling
longitudinal course of bipolar disorder have been noted and serve as a heuristic, nonhomologous model for understanding the development of certain mood disorders (46). In summary, although a given ECT treatment elicits seizure activity, the net outcome is an antiseizure effect over a course of therapy. This may be related to:
Enhanced GABA-ergic transmission
Decreased cerebral blood flow and cerebral metabolic rate (CMR)
Alteration in neuroplasticity
Neurotrophic effects
Neurogenesis
Those who are at high risk for suicidal behavior (27)
Those who are rapidly deteriorating, either physically or psychologically, or both
Those who have a prior history of good response to ECT or a poor history of response to pharmacotherapy
rate to ECT in this group of psychotic patients, however, was 88.2% (i.e., 15 of 17), which compared favorably with the 50% response rate reported for pharmacological treatment (63).
Mania (especially manic delirium)
Schizophrenia
Catatonia associated with major mood disorders, schizophrenia, or organic mood disorders (e.g., systemic lupus erythematosus) (65)
Certain medical disorders
Intractable seizures
Parkinson disease
Hypopituitarism
Neuroleptic malignant syndrome (NMS)
data indicated that ECT was clinically effective while resulting in a similar mortality rate to that with specific drug therapies and approximately half that seen with supportive treatments. Although the specific drug-supportive therapy difference was statistically significant, the sample size in the ECT group was too small (i.e., 28) to ascertain statistical significance.
Space-occupying supratentorial cerebral lesions
A recent history of myocardial infarction and associated instability (<3 months)
Recent intracerebral bleeds
Bleeding or unstable aneurysm or arteriovenous malformations
Retinal detachment
Pheochromocytoma
American Society of Anesthesiologists (ASA) classification risk level of 4 or 5
TABLE 8-1 EFFICACY OF REAL ECT IN COMPARISON WITH SIMULATED ECT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
difference favoring BILAT ECT over UND ECT (i.e., only a 7% overall difference). Of note, subsequent data indicate that high-energy (250% to 600% of seizure threshold) UND ECT approached efficacy rates achieved with BILAT ECT (97,98).
TABLE 8-2 EFFICACY OF ECT IN COMPARISON WITH PLACEBO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 8-3 EFFICACY OF ECT IN COMPARISON WITH TCAs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 8-4 EFFICACY OF ECT IN COMPARISON WITH MAOIs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 8-5 EFFICACY OF BILATERAL ECT IN COMPARISON WITH UNILATERAL NONDOMINANT ECT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 8-6 STATISTICAL OVERVIEW OF STUDIES COMPARING UNILATERAL NONDOMINANT ECT WITH BILATERAL ECT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
to treat until optimal improvement is obtained with ECT. Maintenance strategies to consider in this group include
Antidepressants that have demonstrated at least partial benefit in the past
Lithium or lithium-antidepressant combinations, both in bipolar and unipolar disorders
Combined antidepressant plus antipsychotic (e.g., olanzapine plus fluoxetine for bipolar depression)
Second-generation antipsychotic monotherapy (e.g., quetiapine)
Anticonvulsants, such as lamotrigine, VPA, or CBZ, with or without other mood stabilizers
Maintenance ECT, possibly combined with a mood stabilizer, antidepressant, or antipsychotic
Possibly VNS or TMS
Raising gastric pH before the procedure with a nonparticulate antacid such as sodium citrate because gastric emptying is prolonged in pregnancy
Intubating after the first trimester with a small cuffed endotracheal tube and using a small laryngoscope and a laryngoscope blade to reduce the risk of bleeding from attempted intubation
Elevating of the patient’s right hip and displacing the uterus to the left to reduce the likelihood of
aortocaval compression during the later stages of pregnancy to prevent reduction in fetal circulation
Pretreating with intravenous hydration without glucose to avoid osmotic diuresis
Avoiding hyperventilation because this may hinder oxygen unloading from maternal to fetal hemoglobin
63% for depression
80% for mania
42% for schizophrenia
80% for catatonia
controlled trial comparing ECT with lithium for acute mania, noting that the UND ECT patients did not respond. At that point, the design was changed and all subsequent patients assigned to the ECT group were given the BILAT administration. Final results of this study indicated that the BILAT ECT group tended to demonstrate greater improvement in comparison with the lithium group over an 8-week period (see Chapter 10, “Alternative Treatment Strategies: Electroconvulsive Therapy”) (122). Although these data support the preferential use of BILAT ECT over UND ECT in the treatment of acute mania, this recommendation is not accepted by all. For example, Mukherjee and Sackeim (123) reported no difference in efficacy with either method. Because the ST may be lower in manic patients, the difference in efficacy between BILAT and UND electrode placement may not be as great as for patients with major depression (123,124 and 125). Since these patients often constitute an acute emergency, starting with BILAT ECT may maximize the chances for a rapid resolution of the manic episode. In this context, Karmachary et al. (126) argue that ECT is the treatment of choice for manic delirium.
Advances in anesthetic techniques
Selected stimulus electrode placement (e.g., high-energy UND vs. BILAT)
Change from sinusoidal to brief-pulse or ultrabrief-pulse inducing currents
A better understanding of the role played by the electrical stimulus itself and the degree to which the ST is surpassed
Improved assessment for the adequacy of seizure activity
A complete physical and neurological examination
Routine hematological indices (e.g., complete blood count) and serum electrolytes
Review of cardiac status and electrocardiogram
Simple tests of cognitive function (e.g., MMSE)
Anticholinergic agents (e.g., atropine or glycopyrrolate)
Anesthetic agents (e.g., propofol, etomidate)
Muscle relaxants (e.g., succinylcholine)
β-Blockers (when indicated) to control blood pressure and heart rate changes (e.g., esmolol or labetalol) (128)
many years, alternative agents that may improve the effectiveness of ECT and enhance safety are increasingly employed.
Delayed cognitive recovery
Suppression of adrenocortical function for 8 hours
Pain on infusion (132)
Increased recovery time
Induction of seizures in epileptic patients
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


