KEY POINTS
Surgical therapy is the only effective and proven therapy for patients with severe obesity (body mass index >40 kg/m2). Bariatric operations prolong survival and resolve comorbid medical conditions associated with severe obesity.
During the years 1999 to 2003, called the Bariatric Revolution in the United States, the availability of a laparoscopic approach for bariatric operations caused major changes in the field, including a massive increase in the number of procedures performed as well as an increased publc and professional awareness and understanding of the field.
Bariatric operations involve either restriction of caloric intake or malabsorption of nutrients, or both. Long-term follow-up is essential before the merits of an operation can be confirmed.
Patients who develop a bowel obstruction after laparoscopic gastric bypass require surgical and not conservative therapy due to the high incidence of internal hernias and the potential for bowel infarction.
Malabsorptive operations are highly effective in producing durable weight loss but have considerable nutritional side effects. Patients undergoing such procedures require complete follow-up and must take appropriate nutritional supplements.
The Roux-en-Y gastric bypass is the most commonly performed bariatric procedure, whereas the sleeve gastrectomy is the most rapidly increasing procedure worldwide.
All bariatric operations are tools that serve to allow the patient to lose weight, become healthier, and improve their quality of life. These changes are only maintained long-term if the patient permanently adopts the new eating patterns and exercise habits that are taught and expected in the early year(s) after surgery.
Bariatric surgery is also metabolic surgery, treating the varied metabolic consequences of the comorbid diseases arising from severe obesity. Some operations are particularly effective treatments for such metabolic consequences, such as gastric bypass for type 2 diabetes.
INTRODUCTION
The surgical treatment of obesity has evolved to focus more specifically on the treatment of medical comorbidities associated with obesity than simply obesity itself. While bariatric surgery remains the overriding name of the field, the importance of treatment of the metabolic aspects of obesity has caused the primary society of surgeons treating these problems to rename the society the American Society for Metabolic and Bariatric Surgery (ASMBS). The nomenclature reflects the new emphasis of treating the metabolic consequences of obesity surgically. For the first time in the history of bariatric surgery, considerable effort is being devoted to scientifically study the physiologic mechanisms that help promote weight loss and, more importantly, resolution of comorbid medical problems associated with obesity.
Other major changes in the field of bariatric surgery in the United States since the last edition of this text include the introduction and rapid adoption worldwide of the laparoscopic sleeve gastrectomy and the simultaneous decreasing popularity of the laparoscopic adjustable gastric banding procedure.
The bariatric surgery community also has focused on improvement of outcomes and treatment for patients. The Centers of Excellence (COE) concept is an obvious example in the United States, but internationally, greater attention to outcomes and optimal results has been uniform. Improved outcomes have been documented in the literature, as have notable prospective randomized trials of medical versus surgical therapy. In the United States, a very recent study has challenged the concept that improved outcomes arise in COEs, citing data that Medicare patients had similar complication rates after surgery before and after the institution of a policy of surgery approval only at COEs.1
Extension of the indication for metabolic surgery to patients with class 1 obesity (body mass index, 30–35 kg/m2) has been another development during the past few years.2 The regulatory and scientific communities have been more willing to acknowledge the efficacy of metabolic surgery for treating comorbid medical problems, such as diabetes, than have the payors. Insurance coverage for appropriately qualified patients remains one of the biggest barriers to the access of appropriate care for patients suffering from morbid obesity.
Acknowledgement of morbid obesity as a disease has recently been adopted by the American Medical Association.3 This comes years after the recognition by the Centers for Medicare and Medicaid Services (CMS) of obesity as a disease,4 a reversal of the usual situation in which medical organizations are ahead of payors in recognition of such issues. Despite CMS recognition, regional administrators of CMS still raise unnecessary barriers and make access to care for morbidly obese patients difficult. Long waiting periods, denials for arbitrary issues, and mandatory 6- to 12-month “diet” plans with no requirement for actually losing weight continue to dominate the landscape of the patient seeking metabolic and bariatric surgery. What is also becoming as big an issue for other patients who have had bariatric surgery is denial of subsequent coverage for any emergency complications of the original operation if the patient’s new insurance plan does not cover bariatric surgery. Although the new Affordable Care Act will potentially eliminate the inability to obtain insurance because of pre-existing conditions, there is no guarantee that patients who have had bariatric surgery will be afforded the same rights to payment for emergent and needed surgery should a subsequent complication arise.
The merger of the COE systems of the ASMBS and the American College of Surgeons (ACS) has been a major positive event in recent years as well. Thanks to the leadership of Robin Blackwell, M.D., ASMBS President from 2011 to 2012, supported by the ASMBS Executive Council, the ASMBS took the initiative to merge with the ACS to create a single uniform system acceptable to both groups and clearly more authoritative due to its singular position. The ACS, to its credit, provided large fiscal and human resources to support the merger and adoption of a single COE system. Since the merger, the incorporation of virtually all the centers in the two systems has occurred under one new system. Outcomes, as reported for review by the oversight committee, are better than ever. Our patients have thus been well served.
THE DISEASE OF OBESITY
Obesity is the second leading cause of preventable death in the United States, currently outdone only by smoking. However, obesity as a separate disease entity is still underappreciated and certainly misunderstood. The fact that the American Medical Association waited until the summer of 2013 to acknowledge obesity as a disease entity illustrates this statement.
Obesity is a disease and is likely multifactorial in its origin. We may come to a simpler and direct understanding of its pathogenesis in the future with increased understanding of molecular genetics, but for now, it remains a complex issue. The components of the disease likely include a combination of environmental and genetic factors. The recent rapid rise in the incidence of obesity in less than a generational time suggests that genetic etiologies alone cannot be responsible for the disease. Nevertheless, the numerous factors contributing to the disease increase the difficulty in understanding its causes.
The degrees of obesity are defined by body mass index (BMI = weight [kg]/height [m]2), which correlates body weight with height. Controversy exists as to the most accurate system by which to classify obesity. BMI has its inaccuracies, especially for the heavily muscled individual who may weigh more but have a low amount of body fat. However, BMI is the clinically easiest system to use, employing the simply measured parameters of height and weight. The World Health Organization classification of obesity is given in Table 27-1. It should be noted that for Asian populations, classifications remain the same as the international classification, but the public health action points for interventions are set at 23, 27.5, 32.5, and 37.5 kg/m2.
| CLASSIFICATION | BMI (KG/M2) | |
|---|---|---|
| PRINCIPAL CUT-OFF POINTS | CUT-OFF POINTS FOR ASIANS* | |
| Normal range | 18.5–24.9 | 18.5–22.9 23.0–24.9 |
| Pre-obese | 25.0–29.9 | 25.0–27.4 27.5–29.9 |
| Obese class I | 30.0–34.9 | 30.0–32.4 32.5–34.9 |
| Obese class II | 35.0–39.9 | 35.0–37.4 37.5–39.9 |
| Obese class III | ≥40.0 | ≥40.0 |
Severe obesity is reaching epidemic proportions in the United States and dramatically increasing throughout the rest of the world. Since 1960, surveys of the prevalence of obesity have been conducted every decade by the National Center for Health Statistics. Data on obesity statistics have been updated annually since 1985. The latest figures for obesity incidence in the United States are that 35.7% of U.S. adults are obese (class 1 or higher).5
Genetic and environmental factors contribute to the development of obesity. While children of parents of normal weight have a 10% chance of becoming obese, the children of two obese parents have an 80% to 90% chance of developing obesity by adulthood. The weight of adopted children correlates strongly with the weight of their birth parents. Furthermore, concordance rates for obesity in monozygotic twins are doubled compared to others.6 Diet and culture are important factors as well; these environmental factors contribute significantly to the epidemic of obesity in the United States since the rapid increase in obesity during the past two decades cannot be explained by any genetic etiology.
Other factors appear to contribute significantly to severe obesity. Intermittent or consistent excessive caloric intake occurs. The lack of satiety, on a consistent or intermittent basis, appears to be strongly correlated to such episodes of excessive caloric ingestion. As yet, the physiologic basis for the explanation of such lack of satiety is not understood. Other factors commonly suggested to play a role in the disease of obesity include decreased energy expenditure from reduced metabolic activity, reduction in the thermogenic response to meals, an abnormally high set-point for body weight, or a decrease in the loss of heat energy. Another factor that may influence absorption of ingested food is intraluminal bacterial composition of the intestinal tract. Studies have documented a difference in the composition of the intestinal flora of obese versus normal-weight individuals.7
Obese individuals have excessive adipose cells, both in size and number. The number of such cells often is determined early in life; adult-onset obesity is largely a product of increase in adipose cell size. Weight gain results from increase in both adipose cell size and number. Adipose tissue may be deposited in large quantities in the subcutaneous layer of the abdominal wall or the viscera. Males tend to have central visceral fat distribution, whereas females more often have a peripheral or gluteal fat distribution. Central or visceral fat distribution is associated with metabolic diseases such as diabetes, hypertension, and the metabolic syndrome.8
The severely obese patient often presents with chronic weight-related problems, detailed below. However, the single most difficult aspect of the disease of severe obesity for those suffering from it is the discrimination they face from the rest of the population with respect to social stigmatization. This prejudice against obesity remains the last unlegislated discrimination in existence. Obese individuals are routinely discriminated against in terms of employment. Public facilities often do not allow them to participate in activities. Examples include the size of airline seats and bathrooms, the availability of appropriate clothing options, and the size of automobile cabins. Severely obese individuals are thought of by much of the public as being lazy or gluttonous and lacking self-discipline. They often endure not only discrimination and prejudice, but outright ridicule and disrespect. Consequently, the stigma of severe obesity has a major impact on social function and emotional well-being. Thus, psychological disorders such as depression are found in an extraordinarily high incidence in this population versus the general public. Poor self-image is almost universal among obese individuals.
Significant comorbidities, defined as medical problems associated with or caused by obesity, are numerous. The most prevalent and acknowledged of these include degenerative joint disease, low back pain, hypertension, obstructive sleep apnea, gastroesophageal reflux disease (GERD), cholelithiasis, type 2 diabetes, hyperlipidemia, hypercholesterolemia, asthma, hypoventilation syndrome of obesity, fatal cardiac arrhythmias, right-sided heart failure, migraine headaches, pseudotumor cerebri, venous stasis ulcers, deep venous thrombosis, fungal skin rashes, skin abscesses, stress urinary incontinence, infertility, dysmenorrhea, depression, abdominal wall hernias, and an increased incidence of various cancers such as those of the uterus, breast, colon, and prostate.9
Obesity has a profound effect on overall health and life expectancy, largely secondary to weight-related comorbidities. It is estimated that a severely obese male at age 21 will live 12 years less and a woman 9 years less than a nonobese individual. The incidence of severe obesity in the population is comparable for females below and above the age of 50 years, whereas for men, it is decreased above age 50. This is due to the fact that the severely obese man often is dead of comorbid medical conditions, especially cardiac arrhythmias and coronary artery disease, by age 50. A study carried out by the Veterans Administration showed a 12-fold increase in mortality among 200 morbidly obese men age 25 to 34 years and a six-fold increase in mortality among those age 35 to 44 years over a 7-year follow-up period.10 Decreased quality of life also results due to severe obesity. Most patients seeking surgical treatment of severe obesity do so because of the medical issues they face from comorbid conditions or the decreased quality of life they are experiencing as a result of severe obesity. Bariatric surgery can significantly prolong the lifespan of a severely obese individual, as well as improve the quality of that life.
MEDICAL MANAGEMENT
Medical treatment for severe obesity is aimed at reducing body weight with a combination of decreased caloric intake and accompanying increases in energy expended from moderate exercise. This method of weight loss is the safest possible and may work well for obese individuals who have modest amounts of weight to lose to regain normal body weight or return to being simply overweight instead of obese. However, for the severely obese individual, who usually must lose at least 75 or more pounds to achieve elimination of obesity, this daunting task is extremely difficult. The success rate for the severely obese patient population that tries dieting and exercise as a means of losing enough weight to no longer be obese and maintaining that weight loss is only approximately 3%.
Although the success rate is limited with diet and exercise alone, all severely obese individuals are asked to attempt this route of weight loss prior to undertaking any surgical therapy. There are two main reasons for this. The first is to allow those who can achieve such weight loss through the safest possible means to do so. The second, and by far the most practical, is to have the severely obese individual begin to appreciate and practice the lifestyle changes that must ultimately become routine for them once weight loss is achieved, by whatever means. Thus the dieting and exercise of conservative weight loss plans are important preparation for the incorporation of such habits into the patient’s lifestyle postoperatively after bariatric surgery. The adjustment of the patient’s lifestyle to include these measures is the key to long-term success with any bariatric operation. Failure to do so usually results in regain of weight and sacrifice of any health benefits initially gained by the immediate postoperative weight loss.
The treatment of severe obesity should be initiated with simple lifestyle changes, including moderate reduction of caloric intake and beginning an exercise plan. Walking is the most common choice for this patient population, who may be unable to perform extremely vigorous exercise initially. Medical comorbidities must be identified and treated. Usually the patient’s primary care physician will have already accomplished this, but we do at times identify bariatric-related comorbidities on initial history and physical examination in our clinic.
The severely obese patient will usually have been given dietary counseling by his or her primary care physician and often placed on a medically supervised diet. Most patients also have attempted commercially sponsored diets and diet plans. Success following these is not uncommon, but sustained weight loss for more than a year after stopping the program is uncommon. While primary care physicians usually do an outstanding job of identifying and treating comorbid medical problems, their offices usually do not have the related support staff in terms of nutritionists and psychologists, who are often very helpful in providing services to the severely obese patient undergoing significant lifestyle changes.
Lifestyle changes of diet, exercise, and behavior modification constitute the first tier of therapy for obesity. Dietary restriction and exercise can each independently create a caloric deficit. A daily energy deficit of 500 kcal/d, resulting in a weekly deficit of 3500 kcal, results in the loss of 1 lb of fat weekly. It has been shown that low-calorie diets (800–1500 kcal/d) are as effective as very-low-calorie diets at 1 year, but result in a lower rate of nutritional deficiencies.11 Such diets may produce an average of 8% body weight loss over a 6-month period. Longer follow-up shows recidivism. Physical activity of a moderate daily nature can produce a 2% to 3% body weight loss.12
A behavioral modification program, which provides desirable rewards for meeting short-term dietary or exercise requirements, was shown in combination with diet and exercise to produce as much as a 10% weight loss at 6 months in one study. This weight loss was only sustained in 60% of patients at 40 weeks,13 and at 1 year, the average sustained weight loss was decreased to 8.6%.14
Dietary, exercise, or behavior modification therapy is appropriate treatment for patients who are overweight (BMI <30 kg/m2) and highly recommended for patients with a BMI between 30 and 35 kg/m2. Most of the studies looking at dietary therapy do not include a patient population that is largely obese (BMI >30 kg/m2). Dietary therapy can be effective in producing improvements in comorbid conditions such as diabetes mellitus, with weight loss of 2.3% to 3.7% influencing the disease.15 Thus, lifestyle changes can be effective in improving the health of the nonobese, but less so for the obese population.
Pharmacologic therapy is also an option for patients attempting to lose weight. Unfortunately, the number of effective pharmacologic agents is small compared to the number of products sold with allegations that they will promote or support weight loss. Pharmacotherapy is normally used only after lifestyle changes and dietary therapies have failed. It is used either as primary therapy alone or in conjunction with simultaneous diet and exercise therapy.
Orlistat inhibits gastric and pancreatic lipase enzymes that promote lipid absorption in the intestine.16 It produces a weight loss of between 6% and 10% of body weight after 1 year, but cessation of the drug usually results in prompt regain of lost weight.16 Pharmacotherapy is recommended as an adjunctive or supplementary therapy to lifestyle changes including diet and exercise or behavioral therapy by the National Institutes of Health (NIH) consensus guidelines for treatment of obesity.17 Two new medications have been approved by the Food and Drug Administration (FDA) within the past few years for weight loss. Qsymia, a combination of phentermine and topiramate, produced a 5% weight loss in over 70% of patients after 1 year.18 Lorcaserin (Belviq), a central serotonin agonist, produced a 5% weight loss in 47% of patients taking the medication.19
Since medical therapies are almost uniformly ineffective long-term for patients with severe obesity, the severely obese patient tends to continue to gain weight over time. The number and strength of prescribed medications slowly increase as the medical comorbidities become increasingly worse. Unfortunately, for the majority of severely obese patients, this process continues unabated until death results eventually from the comorbidities. Until recently, less than 1% of severely obese patients underwent surgical treatment for obesity annually. That number has now increased, but still is not over 2% of individuals. Even accounting for patients referred for surgery and felt to be poor candidates, it is likely that less than 5% of patients with severe obesity are referred on an annual basis. Part of this issue may be patient aversion to surgical therapy. Primary care physician awareness, patient reticence, and economic concerns about potentially losing employment due to time off required for surgery or its complications have been the primary reasons cited for the lack of increased performance of bariatric and metabolic surgery. Lack of insurance coverage by many patients is also a primary reason for not seeking surgical therapy for the medical problems associated with severe obesity. Disincentives posed by insurance companies, such as required dietary periods that clearly have no scientific or clinical basis of merit, are still used to discourage patients from pursuing surgical therapy. The lack of efficacy and potential harm to patients of such policies has been clearly demonstrated in the literature.20,21
OVERVIEW OF BARIATRIC SURGERY
Bariatric operations produce weight loss through at least two mechanisms and probably many more that are not known. The most common is restriction of intake. Malabsorption of ingested food is the second mechanism. Restrictive operations may include no or only a modest malabsorptive component. Malabsorptive operations may have some restrictive component, but it is secondary to the malabsorptive aspect of the operation. Table 27-2 describes the common currently performed operations listed by mechanism of action.
This chapter focuses on the operations listed in Table 27-2, whether they are done via a laparoscopic or an open incision approach. Any other procedures to produce weight loss are so infrequently done that they will not be discussed in detail. Vertical banded gastroplasty (VBG), although still one of the approved operations for the surgical treatment of severe obesity based on the NIH Consensus Conference of 1991,22 is now so infrequently performed and has such poor long-term follow-up results23 that it is of historic interest only.
During the 1950s, operations were first performed to treat severe hyperlipidemia with associated obesity.24 These were ileocolic bypass operations to limit absorption and were associated with severe nutritional complications and liver failure postoperatively. The jejunoileal bypass was then devised and popularized in the mid-1970s.25 It was also a malabsorptive operation, but bypassed only a portion of the small intestine. Complications after this procedure included severe diarrhea, electrolyte disturbances, protein-calorie malnutrition, renal stones, and liver failure.
In 1969, Mason and Ito performed the first gastric bypass, describing a loop of jejunum connected to a transverse proximal gastric pouch.26 Bile reflux esophagitis was severe postoperatively, causing Griffin and colleagues to describe the Roux-en-Y modification of the gastric bypass in 1977.27 The gastric pouch was altered from transverse to vertical using the upper lesser curvature as well.
During the 1970s, the dismal failure of the jejunoileal bypass resulted in a very bad reputation in general for bariatric surgery among general surgeons who had performed the procedure or cared for patients suffering from its complications. The reputation was not enhanced by the many variations of gastric stapling that then became common during the 1970s and early 1980s. Such operations often consisted of a few rows of staples partially across the upper stomach for restrictive purposes. Since the procedure was easily done, some surgeons performed it in prolific numbers. Staple breakdown predictably occurred months to years later, with subsequent weight regain. These procedures added to the string of failures of bariatric operations.28
In 1980, Mason29 first performed the VBG, which was a restrictive procedure using a stapled proximal gastric pouch of the upper lesser curvature of the stomach with a restrictive band for its outlet to the rest of the stomach. This operation produced excellent initial weight loss (50% of excess weight or more) with low morbidity and mortality. It rapidly became the most commonly performed bariatric operation in the United States during the 1980s. However, by the early 1990s, it became clear that patients who underwent VBG tended to adopt a diet of high-calorie liquids and regained weight.30 A significant incidence of stenosis at the band was also a problem.31 Long-term weight loss was poor,23 and by the 1990s in the United States, Roux-en-Y gastric bypass (RYGB) became the procedure of choice for bariatric surgery.
In Italy, in the meantime, Scopinaro had developed and popularized the biliopancreatic diversion (BPD) in the early 1980s.32 This procedure, described in more detail later, has been, along with its modification to include duodenal switch (DS),33 the only major malabsorptive operation to enjoy long-term success. BPD and DS are both still used by a fairly select few surgeons throughout the world and have traditionally represented and continue to represent less than 5% of operations performed in the United States.
Fixed banding of the stomach, besides the VBG, was also described by other surgeons in the 1980s and 1990s. Kuzmak is credited with describing the fixed gastric band procedure, which later led to the development of the adjustable gastric banding operation.34
The laparoscopic alternative to bariatric surgery became available in the 1990s. Belachew performed the first laparoscopic adjustable gastric banding (LAGB) operation in 1994.35 Wittgrove and Clark performed the first laparoscopic RYGB the same year.36 Since the former operation is technically much easier than the latter, it was not surprising that it became very popular and frequently performed in Europe and Australia during the late 1990s. In 2001, LAGB was approved for use in the United States. Its popularity increased annually until 2009, but since then, it has decreased in popularity. Sleeve gastrectomy (SG) has enjoyed a rapid increase in popularity both in the United States and internationally since 2008. Buchwald and Oien37 have recently described the international trends in the performance of bariatric operations.
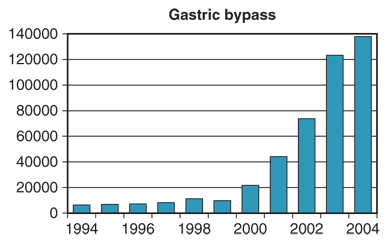
The Bariatric Revolution is a term applied to the 5-year stretch from 1998 to 2003 during which the number of gastric bypass operations performed in the United States, membership in ASMBS, and public recognition and interest and professional respect for the field dramatically increased. Few medical centers in the United States offered a laparoscopic approach to bariatric surgery prior to 1998. Several centers, most notably Schauer and colleagues at Pittsburgh, then began numerous programs to teach many bariatric surgeons the procedure. Simultaneous improvement in the laparoscopic instruments for bariatric procedures combined with these programs to allow the performance of laparoscopic Roux-en-Y gastric bypass (LRYGB) in numerous centers over the next few years. The field then literally exploded in terms of growth in the United States. Figure 27-1 illustrates the volume of LRYGBs performed during the years prior to and during the Bariatric Revolution. Operative procedures increased nearly eight-fold. Membership in the American Society for Bariatric Surgery (the name of the ASMBS at that time) tripled during these years. The number of minimally invasive fellowships offered to graduating residents, most of whom included bariatric surgical procedures as a major component of the case load, increased from about 25 to 125 during these years. Public recognition of the procedure markedly increased due to public figures in the media undergoing bariatric surgery. The widespread availability of the Internet to prospective patients allowed much more information to be relayed. Patients who had or were contemplating surgery were able to communicate via the Internet. Videos of bariatric operations became available via several media outlets including television and the Internet. Many U.S. surgical departments that had previously shunned the field of bariatric surgery recruited bariatric surgeons and established programs. Hospitals without programs recruited surgeons so they could offer the service. Bariatric surgery presentations at national surgical meetings became common instead of rare.
The indications for performing bariatric surgery remain as described in the NIH Consensus Conference of 1991.22 The only major difference is the procedures that are now recognized as standard procedures. These were defined by the CMS in 2005 as those listed in Table 27-2, with the exception of SG. SG did not receive approval until 2012, when after a controversial initial rejection of coverage, the CMS decided to allow coverage based on the discretion of regional carriers. The standard and somewhat conservative indications for performing bariatric surgery are summarized in Table 27-3.
Patient must have: 1. Body mass index ≥40 kg/m2 with or without comorbid medical conditions associated with obesity 2. Body mass index 35–40 kg/m2 with comorbid medical conditions In addition, it is expected that the patient: 3. Has failed attempt at medically supervised diet 4. Be psychiatrically stable |
Of all the reasons a patient who desires bariatric surgery cannot have an operation, insurance coverage, or lack thereof, is the most common reason. This factor, however, is normally beyond surgeon control. Assuming the patient does have coverage or financial means to qualify for surgery, then application of the NIH criteria is the next consideration. Once a patient qualifies according to those criteria, then consideration of medical, social, and psychological issues can determine reasons to avoid surgical therapy.
The NIH criteria do not set limits for age, and surgeon opinion on this issue varies widely. Surgeons have variable practice patterns as to performance of surgery in the older patient. The philosophy for age restriction is two-fold: A large number of younger patients are interested in and eligible for bariatric surgery, and there is a greater likelihood that a longer period of postoperative benefit in terms of improved quality of life and longevity will occur with a younger patient population. Alternatively, older patients are more likely to have debilitating comorbid conditions and thus have immediate benefit in quality of life but not necessarily enhanced longevity.
Medical issues that preclude patients from being good surgical candidates include American Society of Anesthesiologists class IV disease of a nature that makes surgical therapy extraordinarily high risk. Psychological instability or the inability to understand the implications of the proposed operation and what changes will result from it in terms of the patient’s lifestyle are also contraindications. Known and documented drug or alcohol addiction is a contraindication to surgery. Smoking is a relative contraindication, and requirement of cessation of smoking varies by surgical practice. A poorly controlled eating disorder, especially bulimia, is also a contraindication to surgery. Nonambulatory status is a relative contraindication to surgery, especially if the obesity is so severe that the patient cannot normally do self-care or would not likely be able to do so after surgery. Such patients have excessive morbidity in our experience, and placement in care facilities postoperatively after recovery from surgery is often impossible due to their size and limitations of physical ability. Although hard to determine on a single visit, an accumulation of evidence that suggests the patient views the operation as a “magic bullet” for which they must only show up and after which they will not be required to make any substantive changes in eating or lifestyle is a potential valid reason to deny surgery. Finally, lack of sufficient social support, an extremely poor or unsupportive home environment, or hostile spouse or relatives can be contraindications to surgical care, since such supportive environmental factors are important to optimize outcomes once discharged from the hospital. Table 27-4 summarizes potential contraindications for surgery.
1. Severe medical disease making anesthesia or surgery prohibitively risky (American Society of Anesthesiologists class IV) 2. Mentally incompetent to understand procedure 3. Inability or unwillingness to change lifestyle postoperatively 4. Drug, alcohol, or other addiction 5. Active problem of bulimia or other eating disorder 6. Psychologically unstable 7. Nonambulatory status 8. Unsupportive home environment |
PREOPERATIVE ISSUES
Patient selection for surgery should be based on a multidisciplinary team evaluation. The patient has usually been screened by his or her primary care physician prior to referral, with major medical issues addressed prior to referral. Qualification by NIH criteria and availability of insurance coverage can be handled by office administrators to document and confirm prior to the first appointment.
The preoperative assessment of the patient for bariatric surgery must include input from the nutritionist as an important independent evaluation. Careful assessment of the patient’s eating habits, knowledge, self-awareness, and insight are important. An estimation of the patient’s motivation to change eating habits is important. The nutritionist should have at least one assessment session with the patient and an educational session preoperatively once the decision to proceed with surgery has been determined. The operation to be performed requires specific nutritional counseling and education.
Psychological assessment is required by most insurance carriers. One major benefit of the psychological assessment is to determine the patient’s understanding of the operation and whether he or she has a realistic understanding of the changes that are needed in lifestyle for optimal outcomes. The psychologist or psychiatrist often may diagnose previously unappreciated depression, which is prevalent in nearly 40% of our preoperative patients when carefully screened. Its treatment is felt to improve postoperative outcomes.
Most of the patients referred for surgery who have been screened for qualification by NIH standards will be appropriate candidates for bariatric surgery. Some will decide against the procedure after thorough educational and counseling sessions. Such sessions are imperative for improving outcomes. Providing both written detailed information and a verbal presentation by the multidisciplinary team to educate patients preoperatively regarding bariatric surgical procedures and expected outcomes and potential complications is recommended. Informed and prepared patients will likely be much more compliant with perioperative and postoperative requested behavioral and eating changes. Some patients who decide against the procedure will return in the future after their obesity comorbidities worsen. Reevaluation for continued appropriateness as a good surgical candidate is equally indicated at such time.
Preoperatively, current comorbid and other medical problems and their optimal therapy are confirmed. Potentially undiagnosed medical problems are sought. Screening for “hidden” diseases such as coronary artery disease in those patients over age 50 is important. For such patients, or those with known cardiovascular disease, a preoperative cardiology consultation is recommended. Such consultation usually then involves electrocardiogram (ECG), echocardiogram, and stress test, with the results of these at times suggesting need for cardiac catheterization. Another condition often underdiagnosed preoperatively in the severely obese patient population is obstructive sleep apnea (OSA). The Epworth Sleepiness Scale, a standard set of questions evaluating daytime sleepiness, is often used as a screening tool for OSA.38 Many institutions are routinely employing it for all surgical patients, given the recognition that OSA increases postoperative morbidity after all operations. Patients who give a history of loud snoring, morning tiredness on waking, and falling asleep easily while driving or sitting likely have OSA. A diagnostic sleep study is indicated for these individuals. One report suggests that the incidence of sleep apnea in severely obese patients, when all are routinely subjected to sleep studies, may approach 80%.39 Once diagnosed with sleep apnea, patients should use their positive airway pressure appliance as treatment. Use of this system in the immediate postoperative period is especially important to prevent episodes of hypoxia and potentially resulting cardiac arrhythmias. Asthma and hypoventilation syndrome of obesity are other significant pulmonary diseases often requiring preoperative management. Hypoventilation syndrome of obesity is defined as resting arterial partial pressure of oxygen less than 55 mmHg and partial pressure of carbon dioxide greater than 47 mmHg, with accompanying pulmonary hypertension and polycythemia. Pulmonary consultation is indicated for patients with hypoventilation syndrome. Postoperative intensive care unit hospitalization, rarely used after bariatric surgery, may be indicated for these patients.
For patients with active GERD on medication, a preoperative screening upper endoscopy to rule out Barrett’s esophagus and to rule out intrinsic lesions of the stomach or duodenum is recommended. This is especially true for patients planning LRYGB, where the distal stomach and duodenum will be precluded from easy inspection postoperatively. Several studies have documented the considerable incidence of preoperative pathology on flexible upper endoscopy for this patient population, as well as a small but not insignificant incidence of pathology resulting in alteration of the originally planned surgical procedure.40,41 At Virginia, our experience was that such pathology was present in 4.6% of examinations.42 The presence of a hiatal hernia detected on preoperative esophagogastroduodenoscopy will alert the surgeon for the need to perform intraoperative repair.
Patients who are on anticoagulation medication for prosthetic cardiac valves or history of recent venous thromboembolism must have their anticoagulation managed perioperatively. Options include total cessation of oral warfarin therapy 5 days prior to surgery, bridging this time with subcutaneous low-molecular-weight heparin, or, more rarely, preoperative admission for intravenous heparin anticoagulation, which is then stopped 6 hours prior to surgery.
Patients with a strong history of previous venous thromboembolism or who are felt to have multiple risk factors for postoperative venous thromboembolism are potential candidates for preoperative placement of a temporary inferior vena cava filter. Such filters are placed the day prior to surgery by our interventional radiology colleagues and removed 3 to 6 weeks postoperatively. However, a report on a large experience of collected hospitals has shown this procedure has potential complications and morbidity, which may outweigh its use in all but the most high-risk patients.43
Some surgeons routinely perform screening ultrasound of the abdomen for patients planning to undergo LRYGB with an intact gallbladder to rule out the potential presence of gallstones. Should gallstones be discovered, simultaneous laparoscopic cholecystectomy is one option, whereas deferring until after weight loss is another reasonable option. Recent analysis of our experience with simultaneous cholecystectomy at the University of Virginia has shown the average increase in operative time to be approximately 30 minutes for both LRYGB and RYGB with no increase in hospitalization or morbidity.44 An earlier study at Pittsburgh did demonstrate an increased length of hospitalization for patients having simultaneous cholecystectomy.45 When a patient does not have gallstones on preoperative ultrasound, use of prophylactic ursodiol in a dose of 300 mg twice a day will decrease the incidence of gallstone formation after RYGB to approximately 4%.46 Controversy currently exists as to the proper role of simultaneous laparoscopic cholecystectomy at the time of LAGB. The conservative approach is to not perform such an operation, given the small potential for gallbladder bile spillage resulting in an infection of the prosthesis. However, some reports have shown that this risk may be very low.47
Another value of a preoperative ultrasound of the abdomen is the assessment of liver size and composition. An ultrasound that reveals a large fatty liver in a patient planning to undergo either LAGB or LRYGB should alert the surgeon to the high potential for technical difficulty in liver retraction and exposure. We have converted to an open incision and even deferred persisting with the operation altogether when confronted with an excessively large or a tremendously large liver, respectively. Knowledge of this prior to surgery allows the patient to follow a low-calorie diet to shrink the liver preoperatively.
Some surgeons require patients to follow a low-calorie diet in the immediate preoperative period, requiring weight loss prior to proceeding with surgery. Some data exist indicating that preoperative weight loss by patients may improve outcomes.48 Such studies suffer from the fact that the patients who are most compliant and lose weight preoperatively may be the most compliant postoperatively, accounting for the improved outcomes. Preoperative weight loss is not necessary to achieve good outcomes from surgery. Requiring preoperative weight loss raises the dilemma of whether patients who do not achieve weight loss should be then denied the benefit of surgical therapy.
Another controversial area is the requirement for cessation of smoking by patients prior to undergoing surgery. Some surgeons make this an absolute requirement, while others do not. Certainly the risk of marginal ulcer after RYGB, which is more difficult to treat and has a higher recurrence rate in smokers,49 should be considered as a factor in whether to offer the smoking patient this operation.
Other preparation for bariatric surgery includes the performance of a baseline arterial blood gas measurement. This is especially important in any patients with significant pulmonary disease or hypoventilation syndrome of obesity, since a baseline value of “normal” for the patient must be appreciated if ventilator management postoperatively is necessary.
Baseline evaluation of thyroid function is indicated preoperatively, since hypothyroidism is not uncommon in this patient population. Serum chemistries, liver function tests, and usual screening blood tests are done. Blood tests to determine baseline nutritional parameters are not uncommonly abnormal for low iron and vitamin D levels. The former finding is common in the menstruating female population, obese or not, while the latter has been reported for the population in general but especially for the obese patient population.50 The necessity to treat low vitamin D levels preoperatively is unclear in terms of its influence on clinical outcomes. However, the primary reason to correct low vitamin D is improved long-term bone disease health.
Preoperative education is important to reemphasize important points of likely events of the perioperative period, expected postoperative course, and instructions for postoperative activity and diet. Expectations for patients include ambulation on the day of surgery, following postoperative dietary instructions, taking recommended vitamin and mineral supplements, and following a regular exercise plan.
A preoperative anesthesiology assessment is indicated for all patients undergoing bariatric surgery. This assessment confirms the optimal assessment and management of ongoing comorbid medical problems. It also includes the aforementioned cardiopulmonary evaluation to determine any underlying pathology that requires preoperative treatment to decrease perioperative morbidity from cardiopulmonary complications.
It is important that the anesthesiologist be experienced in performing general anesthesia management for the bariatric patient. There is no question that the consistent participation of a few selected and experienced anesthesiologists who specialize in the care of the bariatric patient will minimize morbidity of surgical outcomes. Perioperative and intraoperative communication between the anesthesiology team and the surgical team is particularly important during bariatric operations to facilitate the smooth flow of the operation and avoid complications from the procedure.
Two major difficulties that the anesthesiologist faces when performing a general anesthetic for the severely obese patient are vascular access and airway management. Both are significantly more difficult in the obese patient population than the normal-weight population. Central venous access is at times the only available route for establishment of a reliable intravenous access. Access to both arms during the procedure is standard in our operating rooms; this allows for additional larger bore intravenous access should an episode of intra-abdominal hemorrhage occur. Jugular cannulation is often very difficult due to neck adipose tissue. However, ultrasound guidance can facilitate this procedure. Subclavian access is performed when other central access routes are not feasible.
Management of the severely obese patient airway is often a major challenge for the anesthesiologist and must be done well to avoid potentially significant morbidity. Videotelescopic intubation systems are successfully used in some institutions to manage difficult airway intubation. Fiberoptic laryngoscopy is more commonly used for the difficult airway should standard laryngoscopy provide an inadequate view. For the most difficult class IV or even class III airways, a fiberoptic guided intubation is used. Experience is key in performing smooth intubation of this patient population. Significant preoxygenation for 3 minutes or longer prior to intubation is used for the severely obese patient to provide a longer safe duration for intubation should difficulties be encountered. However, desaturation must be immediately addressed with reestablishment of oxygenated ventilation because this patient group does not tolerate any prolonged desaturation without potential adverse cardiopulmonary consequences.
The anesthesiologist must be adept at understanding and managing alterations in cardiopulmonary function from the use of a pneumoperitoneum during laparoscopic bariatric procedures. These alterations include the effects of carbon dioxide absorption on required minute ventilation, the potential for bradyarrhythmias, and the potential for decreased systemic pH with longer procedures in patients with preexisting cardiopulmonary disease. Arterial monitoring of the latter group of patients may be necessary by the anesthesiology team, and a radial arterial line is standard for such patients.51
Drug pharmacokinetics differ in severely obese patients as well. Changes in volume of distribution include smaller-than-normal fraction of total body water, greater adipose tissue content, altered protein binding, and increased blood volume. Possible changes in renal function and hepatic function must be considered when administering drugs.
Specific anesthetic drug metabolic alterations in the severely obese include a larger volume distribution of thiopentone, resulting in a prolonged effect of the drug.
Calculation of the dosage should be done by lean body weight. Benzodiazepines also exhibit a prolonged elimination phase, causing persistence of their effects. Increased pseudocholinesterase activity is present in the severely obese patient, requiring increased dosages of pancuronium. Enflurane metabolism is increased over the average-sized person, requiring a lower dosage of this agent.
BARIATRIC SURGICAL PROCEDURES
The procedures in this section will be described using a laparoscopic approach as the default or typical approach. RYGB, BPD, and DS may still be performed by some surgeons using an open approach, but this has now become the exception. Minimization of the morbidity of the open incision, especially incisional hernias and wound complications, as well as earlier hospital discharge and lower 30-day complication rates have all been clearly shown to favor using a laparoscopic approach when feasible.52,53,54 In addition, the logical assumption that avoiding the major tissue trauma associated with a lengthy abdominal wall incision is beneficial to patient recovery has been confirmed. Most importantly, patient interest in bariatric surgery increased dramatically once a laparoscopic approach was available for these procedures, especially RYGB.55 In the twenty-first century, most prospective bariatric patients are informed enough about the options for surgery that they seek out a surgeon who does laparoscopic bariatric surgery.
When an open surgical approach is used for any of these procedures, an upper midline incision is the most commonly used approach. Some surgeons have had excellent success with a left subcostal incision for performing RYGB.56 Mechanical retractors afford additional exposure for open surgery and are indicated. Wound closure for midline incisions usually is performed using heavy monofilament suture for the midline fascia, but surgeon preferences vary. Any concerning drainage from a postoperative open surgical incision line requires opening of the wound in that area and confirmation that a more severe deep-seated fascial tissue infection does not exist.
Laparoscopic surgery requires a basic core set of knowledge and skills that have now become a standard part of surgical training. Successful completion of the Fundamentals of Laparoscopic Surgery (FLS) unit developed by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES)57 is now mandatory for all surgical residents in the United States.
Laparoscopy begins with the safe creation of a pneumoperitoneum, often a difficult step in the bariatric patient. We have found the use of a tracheostomy hook inserted through a trocar-sized incision to elevate the fascia in the left subcostal region to be of great assistance in facilitating the insertion of a Veress needle into an appropriate location for pneumoperitoneum creation. In general, the use of a Hasson approach for creating a pneumoperitoneum in the bariatric population is limited by the thick body wall. In the patient with an extremely thick body wall, extra long trocar ports can be used for laparoscopic surgery.
The pneumoperitoneum pressure that is used when performing bariatric surgical procedures is generally in the 15 to 18 mmHg range. A high-flow insufflator is mandatory to maintain the pneumoperitoneum for adequate and safe visualization. An angled telescope is quite helpful. Instrumentation for performing laparoscopic bariatric surgery has dramatically improved in the past 15 years and continues to improve. We now favor using certain laparoscopic instruments, such as the staplers and harmonic scalpel, even if conversion to an open approach occurs.
Conversion to an open incision is appropriate in circumstances where patient safety would potentially be compromised by persisting with a laparoscopic approach. Table 27-5 lists appropriate reasons for conversion to an open incision as well as consideration for beginning with open surgery if certain conditions are known, such as an existing large upper abdominal incisional hernia or known severe intra-abdominal adhesions. Conversion to an open incision should not be viewed as a failure by the surgeon, nor should such an attitude bias the surgeon in favor of persisting with a laparoscopic approach if the operation is not progressing or if a complication is worsening when it could be corrected more quickly using an open approach. Patient safety is the gold standard for determining the timing and appropriateness of conversion. Usually, if conversion is needed, it is best to do so as early in the course of the operation as possible.
1. Failure to establish an adequate pneumoperitoneum 2. Hemodynamic adverse reaction to pneumoperitoneum 3. Intra-abdominal adhesions precluding safe access or presenting excessive difficulty to access abdomen 4. Hepatomegaly such that retraction is not feasible or, even with retraction, organ visualization is obscured 5. Intraoperative complications such as hemorrhage that are best managed with an open approach 6. Exceedingly thick body wall precluding adequate trocar access or manipulation 7. Existing large upper abdominal wall hernia that optimally can be repaired simultaneously using the same incision |
Short-term follow-up is defined as follow-up of up to 2 years. Unfortunately, even in the best of practices in the United States, because of the lack of a centralized health system or registry, 1-year follow-up of 90% or greater is a laudable achievement and rarely reported in most case series. Recommendations for bariatric centers wishing to be COEs are that 75% of patients are followed for 5 years with restrictive operations, and 90% are followed if they have malabsorptive operations. Those recommendations, however, are based on having a system that attempts maximum possible follow-up that should yield such results. Although a system may be in place that generates multiple attempts at having the patient return for postoperative checkups, without patient compliance, all such systems are fallible.
The goals of short-term follow-up are to maximize care of the patient in the postoperative period; assist in adjustment to new eating, exercise, and lifestyle patterns; be on the alert for and treat postoperative complications; and recommend measures to limit such complications. Objective data that should be obtained after all bariatric operations include weight loss, change in BMI, resolution or improvement in medical comorbidities, and any adverse events or complications that occur. Optimally, assessment of quality of life can help gauge efficacy as well, with the Short Form-36 (SF36) questionnaire being one standard, frequently used example. Short-term follow-up data do give a good reflection of the safety of the procedure, but only an estimate of the efficacy regarding weight loss and effect on resolution of medical comorbidities.
Medium-term follow-up is defined as that from 2 to 5 years. Medium- and long-term follow-up, defined as greater than 5 years, are the only means by which the true long-term efficacy of bariatric surgical operations can be assessed. Operations that initially appear quite promising, such as the VBG or even the jejunoileal bypass, were shown with long- and medium-term follow-up, respectively, to have significant deficiencies in efficacy for the VBG23 and safety25 for the jejunoileal bypass. Other stapled gastroplasties similarly did not demonstrate efficacy on medium-term follow-up.25
Unfortunately, to date, there has been no conventional method of reporting outcomes after bariatric surgery. The ideal publication includes quantitation of number of patients, a description of the operative technique, incidence of conversion to open if applicable, number of patients included in follow-up data per year, percentage of patients lost to follow-up, weight loss usually expressed as percentage of excess weight, initial and subsequent BMI, complications, mortality, resolution of medical comorbidities, and any quality of life data. Few publications have met these criteria. There are also only a few studies in which a prospective randomized comparison either between bariatric surgery and medical management or between different bariatric surgical operations or approaches (laparoscopic vs. open) have been performed. Improvement in study design and more complete data in future publications are indicated.
A multidisciplinary team approach to follow-up is as essential, if not more so, postoperatively than preoperatively. Regular counseling sessions with the nutritionist are always helpful. Psychological support should be available as needed to assist the patient in adjustment to major life changes. All programs should offer a frequent support group forum for patients to discuss issues on a less formal basis and receive encouragement from other patients as well as staff.
Experience of performing bariatric surgery for several decades between the co-authors has led us to conclude that whatever the operation performed, its long-term success is only achieved by patients if they embrace the eating and lifestyle changes the operation allows them to adopt. Continuation of exercise as part of the daily lifestyle is associated with a high incidence of preservation of weight loss. Diligence to avoid snacking and returning to other poor eating habits is also important. The majority of patients do embrace the metamorphosis their bariatric operation produces such that they maintain their new eating, lifestyle, and exercise habits to the benefit of their continued improved health, self-image, and well-being.
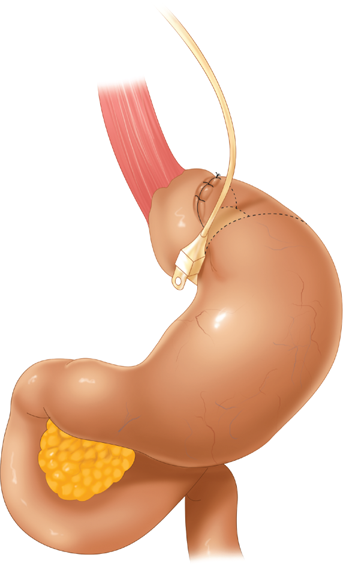
LAGB involves placement of an inflatable silicone band around the proximal stomach. The band is attached to a reservoir system that allows adjustment of the tightness of the band. This reservoir system is accessed through a subcutaneously placed port, similar in concept to ports used for chemotherapy via central venous catheters. Figure 27-2 shows the LAGB apparatus in place.
Two major types of bands have been used for this procedure. The original Lap-Band, most recently marketed by Apollo Endosurgery, has been used most frequently. The Swedish Band, now remarketed as the Realize Band by Ethicon, is slightly wider than the Lap-Band.58 The port systems have differences as to profile and methods of attachment to the fascia.
Port placement for LAGB has varied among surgeons. Figure 27-2 shows a common configuration used. Usually some combination of two ports for the surgeon’s hands, one or two for the assistant, a port for the telescope, and a liver retractor site are needed.
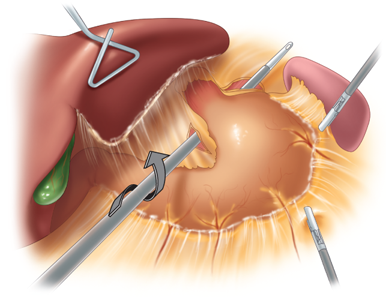
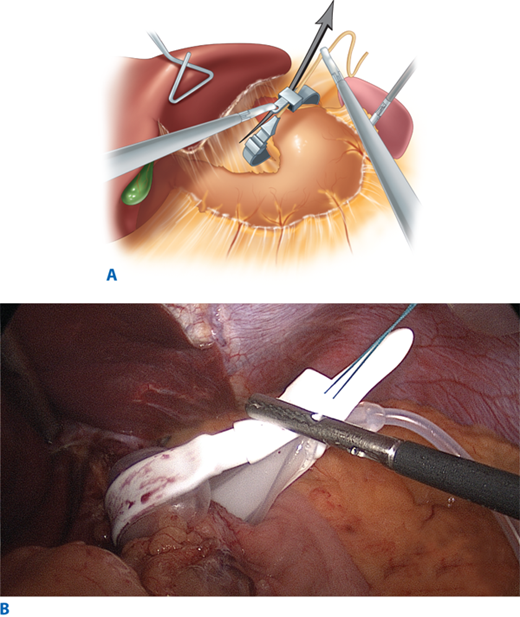
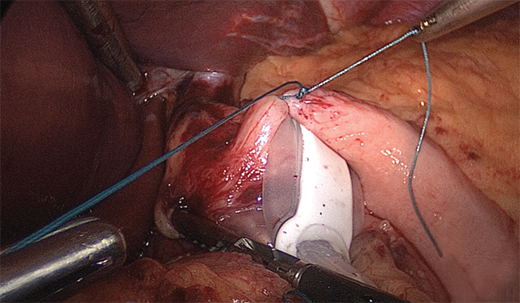
With the patient placed in reverse Trendelenburg position, the procedure begins with division of the peritoneum at the angle of His and then division of the gastrohepatic ligament in its avascular area (the pars flaccida) to expose the base of the right crus of the diaphragm. If a hiatal hernia is present, it must be repaired at this point, using a standard posterior esophageal dissection to expose the crura and perform suture repair. A grasper (Lap-Band) or specially devised instrument (Realize Band) is inserted along the base of the anterior surface of the diaphragmatic crura, from right to left, emerging at the angle of His in the area of the divided peritoneum (Fig. 27-3). The device is then used to pull the band underneath the posterior surface of the gastroesophageal junction. This technique, by passing the band through some fibrous tissue in this plane, serves to anchor the band more securely posteriorly. During the initial years of band placement, a retrogastric location of the posterior half of the band in the free space of the lesser sac caused an unacceptably high incidence of slippage and prolapse of the band. The adoption of the pars flaccida technique decreased the incidence of such slippage.59
Figure 27-3.
Grasper being passed through under stomach to grasp tubing during placement. (From Schauer PR, et al, eds. Minimally Invasive Bariatric Surgery, 1st ed. New York: Springer; 2007. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2005-2009. All rights reserved.)
Once the band is passed around the proximal stomach, it is locked into its ring configuration through its own self-locking mechanism. This involves the tubing end being passed through the orifice of the buckle for the Lap-Band and the suture on the end of the flanged end of the band site being passed through for the Realize Band. Once the band is securely locked in place, the buckle portion of the band is located on the lesser curvature of the stomach (Fig. 27-4A,B). Now the anterior surface of the fundus and proximal stomach is imbricated over the band using several sutures (Fig. 27-5).
Figure 27-4.
A. Lap-Band in place around stomach. (From Schauer PR, et al, eds. Minimally Invasive Bariatric Surgery, 1st ed. New York: Springer; 2007. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2005-2009. All rights reserved. B. Realize (Swedish) Band around stomach.
The tubing of the band system is brought out through the desired site for placement of the port portion of the system. Usually this is a trocar site near the upper abdomen or xiphoid region to place the port most superficially such that it can be palpated postoperatively. The port is secured to the anterior abdominal wall fascia. Access to the port for subsequent addition of fluid to the band system is percutaneously achieved using a Huber or noncutting type needle. The band is initially placed empty of fluid in most circumstances.
Most LAGB procedures are done on an outpatient basis. Technically the operation is not as difficult as the other operations described later. Because the gastrointestinal tract is not violated, the relative risk of the procedure is lower than most other bariatric operations, making this procedure more amenable to offer to older, more medically ill, or higher risk patient populations. However, efficacy of the operation in the superobese (BMI >50 kg/m2) is less impressive, with average BMI remaining over 40 kg/m2 after 5 to 8 years of follow-up.60 It has been our impression that optimal results occur with this operation in a patient population who is motivated, needs to lose less than 50 kg to achieve a BMI less than 30 kg/m2, is willing and able to exercise regularly, is amenable to changing eating patterns as recommended, and is within a geographically close enough area for ease of follow-up. Patients who are impatient to lose weight, immobile, unable or unwilling to exercise, or confirmed “grazers” or “nibblers” on high-calorie sweets who expect to be able to continue their dietary habits without great alteration are not good candidates for this operation. Similarly, patients who have had previous upper gastric surgery, such as a Nissen fundoplication, are relatively poor candidates for LAGB due to the potential tissue compromise in taking down the wrap to place the band.
Important points of preoperative preparation specific to the procedure include being nil per os (NPO), receiving preoperative appropriate venous thromboembolism prophylaxis, appropriate broad-spectrum intravenous antibiotics, and having appropriate intravenous access and monitoring. An orogastric tube is inserted into the stomach. These preoperative measures are recommended in all the procedures described later as well.
The majority of LAGB procedures are performed on an outpatient basis. Insurance requirement and pre-existing medical issues are usually the only reason for overnight hospitalization. Diet instructions, wound care, pain medications, and instructions on time schedule of resuming other preoperative medications should all be explained to the patient as well as to a family member (who has not just undergone general anesthesia) prior to discharge. Arrangements for a postoperative follow-up visit, phone numbers to call for emergencies, and indications to call should all be explained as well.
The first postoperative evaluation after LAGB generally occurs 2 to 3 weeks after surgery. By this time, patients have begun to tire of the blenderized diet recommended, are often eager to return to work if they have not done so, and are amenable to discussions of an exercise plan and diet progression plan. Wounds are assessed, as are medical problems, oral intake, and adherence to diet. Since LAGB does not preclude absorption of any specific nutrients, we only recommend a multivitamin for patients whose preoperative lab values are normal. Use of ursodiol (300 mg twice a day) for gallstone prophylaxis after LAGB is variable from surgeon to surgeon. Weight loss after LAGB often does not occur as rapidly as after gastric bypass, and data regarding gallstone formation after LAGB are lacking. However, rapid weight loss after LAGB will occur in some patients justifying prophylaxis with ursodiol.
Band adjustments and postoperative support group sessions are extremely important for good outcomes after LAGB.61 Band adjustments are paramount as part of the operation. The actual performance of the LAGB procedure is really only a small part of the care of the patient. Frequent postoperative visits, band adjustments as necessary, and participation in an appropriate exercise program are all important for postoperative success for these patients. Recommendations for timing of band adjustments vary from practice to practice. In general, there is agreement that losing less than 2 lb per week is an indication to increase the restriction of the band by adding fluid. Patients who can easily eat most solid foods and have little satiety and a fairly pronounced appetite need additional restriction from the band.
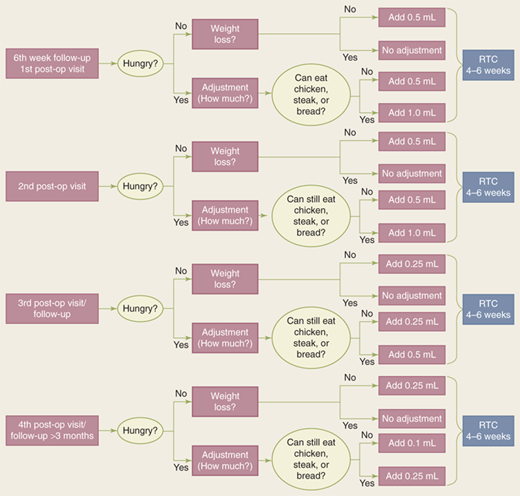
Band fills usually are accomplished in the outpatient clinic setting. Occasionally fluoroscopic radiology is needed to assist in accessing the port, based on the depth of the port from the skin and its ease of palpation. Experience by the person doing the band fill is an important factor in increasing the percentage of patients able to be accessed in clinic without radiology assistance. A careful record should be maintained of the amount of fluid in each patient’s band. Some surgeons will withdraw all the fluid at each fill, reinserting the desired amount. Many will just add additional fluid as indicated. The amount of fluid added is based on hunger, weight loss, and ability to eat meat or bread. Figure 27-6 shows an in-office algorithm adjustment scheme used by Ren and colleagues at New York University.62 Ideally, adjustments are performed over approximately a 2-year period after surgery. However, changing life and clinical circumstances can require adjustments at any time thereafter.
Figure 27-6.
Algorithm for postoperative band adjustment. RTC = return to clinic. (Reproduced with permission from Ren CJ. Laparoscopic adjustable gastric banding: postoperative management and nutritional evaluation. In: Schauer PR, et al, eds. Minimally Invasive Bariatric Surgery. 1st ed. New York: Springer; 2007:200. With kind permission of Springer Science + Business Media.)
An optimal situation for LAGB success is a program whose patients all live within an easy drive of the center, will and do participate in frequently available support groups and use exercise facilities supplied by the program, have access to band adjustment visits as needed, and are carefully selected for appropriateness and motivation for the procedure preoperatively.
Medium- to long-term (8-year follow-up) outcomes have been reported for LAGB from Weiner et al.63 At 5 and 7 years after LAGB, an average of published studies in the literature showed that patients lost 60% and 58% of excess weight, respectively.64 Resolution of comorbidities after LAGB has been reported as overall very good, with hypertension resolving in 55% at 1 year,64 observed sleep apnea decreasing from 33% to 2%,65 GERD improving in over 50% of cases,66 and asthma,67 depression,68 and quality of life69 improving for patients after LAGB. Dixon and colleagues70 published a landmark article describing the vastly superior results of managing patients with diabetes using LAGB versus optimal medical management. Resolution of diabetes was 13% in the medical group versus 73% in the surgical group after a 2-year follow-up.
Large institutional series of LAGB results have been published from centers in Europe and Australia, with good results71,72 for the Lap-Band. Similarly excellent results have been published for the Swedish adjustable band.73 Buchwald and colleagues74 performed a meta-analysis of all bariatric surgical papers published from 1990 to 2003. Overall mortality for LAGB was given as 0.1%. Table 27-6 shows the data from this paper and another meta-analysis75 for weight loss, morbidity, and mortality for LAGB compared with RYGB and the malabsorptive procedures. Table 27-7 also shows data also from Buchwald and colleagues74 regarding the percentage of resolution of four major comorbidities associated with obesity after the most common types of bariatric operations, including LAGB.
Specific complications that may occur after LAGB include prolapse, slippage, erosion, and port and tubing complications. In addition, just plain failure to lose weight is more commonly seen with this procedure than with other common bariatric procedures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree