KEY POINTS
The epidermis consists of continually regenerating stratified epithelium, and 90% of cells are comprised of ectodermally derived keratinocytes.
Dermal fibers are predominantly made of type I and III collagen in a 4:1 ratio. They are responsible for the mechanical resistance of skin.
Staphylococcus aureus is the most common isolate of all skin infections. Impetigo, cellulitis, erysipelas, folliculitis, furuncles, and simple abscesses are examples of uncomplicated infections, whereas deep-tissue infections, extensive cellulitis, necrotizing fasciitis, and myonecrosis are examples of complicated infections.
Hemangiomas arise from benign proliferation of endothelial cells surrounding blood-filled cavities. They most commonly present after birth, rapidly grow during the first year of life, and gradually involute in most cases.
Basal cell carcinoma represents the most common tumor diagnosed in the United States, and the nodular variant is the most common subtype.
Squamous cell carcinoma is the second most common skin cancer, and primary treatment modalities are surgical excision and Moh’s microsurgery. Cautery and ablation, cryotherapy, drug therapy, and radiation therapy are alternative treatments.
Tumor thickness, ulceration, and mitotic rate are the most important prognostic indicators of survival in melanoma. If a sentinel node contains metastatic cancer, prognosis is determined by the number of positive nodes, the primary tumor thickness, mitotic rate and ulceration, and the age of the patient.
INTRODUCTION
The skin is a complex organ encompassing the body’s surface and continuous with the mucous membranes. Accounting for approximately 15% of total body weight, it is the largest organ in the human body. Enabled by an array of tissue and cell types, intact skin protects the body from external insults. However, the skin is also the source of a myriad of pathologies that include inflammatory disorders, mechanical and thermal injuries, infectious diseases, and benign and malignant tumors. The intricacies of this organ and associated pathologies are reasons the skin and subcutaneous tissue remain of great interest and require the attention of various surgical disciplines that include plastic surgery, dermatology, general surgery, and surgical oncology.
ANATOMY AND HISTOLOGY
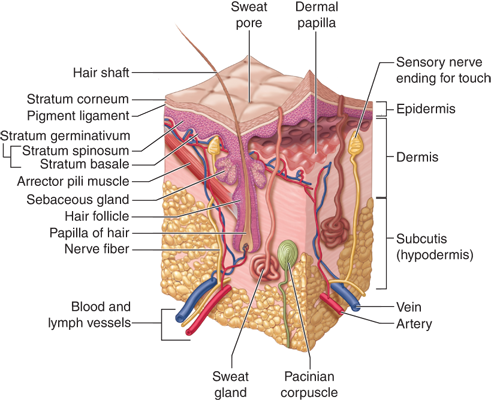
Components of epithelial, connective, vascular, muscular, and nervous tissue are organized into three histologic layers (epidermis, dermis, and hypodermis), which vary in consistency between various body parts (Fig. 16-1). The thickness of each layer, distribution of dermal appendages, density and type of nerve endings, and melanocyte distribution are just some of the variables that differ by location and purpose. The epidermis and its appendages are of ectodermal origin, whereas the dermis and hypodermis are of mesodermal origin.1
The epidermis consists of stratified epithelium that undergoes continuous regeneration. Ninety to ninety-five percent of these epithelial cells are ectodermally derived keratinocytes. During their differentiation, keratinocytes form flattened, anucleate cells that are ultimately shed from the skin surface. This process results in the formation of distinct cell layers (from deep to superficial): stratum basale (single cell layer), stratum spinosum (5 to 15 cells thick), stratum granulare (1 to 3 cells), and stratum corneum (5 to 10 cells), which is further subdivided into a deep, compact stratum compactum layer and a more superficial, loose stratum disjunctum layer. In the palmoplantar region, an additional layer, the stratum lucidum, can be seen between strata granulare and corneum (see Fig. 16-1). Transit time (keratinization) is approximately 30 days. Epidermal thickness differs between skin regions, ranging from 50 μm on the eyelids to 1 mm on the soles. Interventions such as tissue expansion result in thickening of the epidermis (and thinning of the dermis).
Basal layer keratinocytes are columnar or cubical cells with a basophilic cytoplasm and large nucleus, and they are aligned with an underlying basement membrane and anchored by hemidesmosomes. Melanosomes are positioned over the nucleus. The cytoplasm includes bundles of filaments comprised of keratin polypeptides; these insert into the desmosomes and contribute to the formation of the cytoskeleton, conferring mechanical resistance to the epidermis as a whole. Other types of intercellular junctions (including gap and adherens junctions) are present as well. Proliferation occurs at this cell layer. Spinosum layer keratinocytes are polygonal, with an eosinophilic cytoplasm. Ultrastructurally, they contain coarse bundles of tonofilaments, cytoplasmic protein found in epithelial cells.
Granular layer keratinocytes are flattened cells, lying parallel to the skin surface, with a diameter of 25 μm; they contain keratohyalin granules and keratin and lamellar bodies. The latter are involved in the process of desquamation and in the formation of a lipid pericellular coat that acts as a penetration barrier against foreign (hydrophilic) substances.
Corneum layer keratinocytes are highly flattened, hexagonal, eosinophilic cells, containing mainly keratin matrix, that are eventually shed from the skin surface and contribute to the skin’s barrier function. The superficial part of eccrine sweat glands and hair follicles are considered part of the epidermis as well. The epithelial cells comprising these units have separate biologic properties with regard to regeneration, differentiation, and response to various stimuli. Five to ten percent of epidermal cells are nonkeratinocytes, including primarily Langerhans cells, melanocytes, and Merkel cells.
These are mobile, dendritic, antigen-presenting cells present in all stratified epithelia that originate from bone marrow precursors. These cells are capable of uptaking exogenous antigens (by use of Birbeck granules), processing them and presenting them to T cells; they represent 3% to 6% of all cells in the epidermis.2
Originating from the neural crest, these cells migrate into the epidermis where they produce melanin, the main natural pigment of the skin. They are distributed regularly among basal keratinocytes, at a ratio of 1 melanocyte for every 4 to 10 keratinocytes. Their density reaches 500 to 2000 cells per mm2 of cutaneous surface, with regional variations (maximal density on genital skin).
Melanin is produced through the enzymatic activity of tyrosinase on the substrate tyrosine and is then stored in melanosomes; these are transported along the dendritic processes of melanocytes and are eventually transferred to adjacent keratinocytes where they form an umbrella-like cap over the nucleus, protecting it from the effects of ultraviolet (UV) light. Melanocytes express the bcl-2 oncoprotein, S100 protein, and vimentin.
Ethnic variations in pigmentation are due to differences in activity of melanocytes and distribution of melanosomes within the epidermis and not differences in the number of melanocytes.
Merkel cells display both neuroendocrine and epithelial features. They function as mechanoreceptors and synapse with dermal sensory axons in the basal layer of the epidermis and the epithelial sheath of hair follicles.
The normal human epidermis contains a small percentage (<1%) of lymphocytes, present mainly in the basal layer. They express predominantly a T-memory/effector phenotype.3
These are cells with a clear cytoplasm and are located within the nipple epidermis in 10% of both males and females. Their role in normal skin and pathology remains poorly understood, but they may be precursors of Paget’s cell carcinoma.4
These are specialized epithelial structures, connected to the surface epidermis but located mainly within the dermis and hypodermis. Appendages serve functions that include lubrication, sensation, contractility, and heat loss.
Sweat glands are tubular exocrine glands, consisting of a secretory coil and an excretory duct. Eccrine sweat glands are the main sweat glands in humans, playing a vital role in the process of thermoregulation. They are present almost everywhere on the skin (except mucous membranes), with a maximal density over the palms, soles, axillae, and forehead.
Apocrine sweat glands are less abundant in humans and are derived embryologically from the germ cells that produce the pilosebaceous follicle and are, therefore, structurally associated with it. These glands are found in the axillary, anogenital, and nipple regions. They consist of a secretory coil that is larger and more irregular in shape than that of eccrine glands.
A third type of sweat gland was more recently described in the axillary region. The so-called “apoeccrine” glands are atrichial glands, opening directly to the skin surface, but their secretory coil is similar to that of apocrine glands and they present during puberty.5
These structures are derived from the epithelial germ layer and lie obliquely in the dermis, with their deepest part reaching the hypodermis. They are present throughout the integument, excluding the glabrous skin (palms, soles) and portions of the genitalia. Their size and morphology are variable (terminal, vellus, lanugo, and intermediary hair). Their growth is cyclic and proceeds through three distinct phases of uneven duration (anagen, catagen, and telogen) during which their histology varies considerably.
The nails overlie the dorsal aspect of the distal phalanges of the fingers and toes. They consist of three parts: (a) the root, covered by the proximal nail fold, continuous with the lateral nail folds; (b) the nail plate, comprised of hard keratin; and (c) the free edge, overlying the hyponychium, a thickened epidermis. The nail lies on the nail bed, a richly vascular connective tissue containing numerous arteriovenous shunts. The proximal part of the nail bed is continuous with the nail matrix, responsible for nail growth and adhesion.
The dermis is a compressible, elastic connective tissue that supports and protects the epidermis, dermal appendages, and neurovascular plexuses. It consists of cells, fibrous molecules, and a ground substance. It turns over continuously, regulated by mechanisms controlling the synthesis and degradation of its protein components. The thickness of the dermis varies considerably with the anatomic location (being much thicker on the back, palms, and soles than on the eyelids).
The papillary (superficial) dermis forms conic upward projections (dermal papillae) alternating with epidermal rete ridges, thus increasing the surface of contact between the dermis and epidermis and allowing for better adhesion between these layers. It contains several cell types (fibroblasts, dermal dendrocytes, and mast cells), vessels, and nerve endings. It is made of collagen fibers arranged in loose bundles and thin elastic fibers stretching perpendicularly to the dermal-epidermal junction. In the distal extremities, dermal papillae contain tactile corpuscles, specialized nerve endings acting as mechanoreceptors. The reticular (deep) dermis is made of coarser collagen bundles, tending to lie parallel to the skin surface. The elastic network is also thicker in this layer. The reticular dermis contains the deep part of cutaneous appendages and vascular and nerve plexuses.
The majority (>90%) of dermal fibers are collagen, predominantly types I and III, which are responsible for the mechanical resistance of the skin. Collagen accounts for 98% of the total mass of dry dermis. Collagen fibers are arranged in bundles that are loose in the papillary dermis and become thicker in the deep dermis. Other collagens found in the dermis include type IV collagen (at the dermo-epidermal junction and in the basement membranes of cutaneous appendages, vessels, muscles, and nerves) and type VII collagen (anchoring fibers of the dermo-epidermal junction).
Elastic fibers are responsible for the retractile properties of the skin due to their ability to stretch to twice their resting length and return to their baseline shape after the deforming force is relieved. In the papillary dermis, they are thin; they become thicker in the reticular dermis, where they tend to run horizontally. By electron microscopy, elastic fibers show variations depending on age and the area studied (sun-exposed or not). They have irregular contours and are made of a central amorphous matrix composed of elastin, an insoluble protein. This core is surrounded by a varying number of microfibrils made of fibrillin. Reticulin fibers consist biochemically of an assembly of thin collagen fibers (types I and III) and fibronectin.
Fibroblasts are the fundamental cells of the dermis and all connective tissues that synthesize all types of fibers and the ground substance. They appear as spindle-shaped or stellate cells, containing a well-developed rough endoplasmic reticulum. Myofibroblasts are cells derived from fibroblasts, namely during the process of wound healing; they contain myofilaments, visible by electron microscopy, and express (smooth) muscle actin and more rarely desmin.6
Dermal dendrocytes represent a heterogeneous population of mesenchymal dendritic cells, recognizable mainly by immunohistochemistry. They are present around capillaries of the papillary dermis, around sweat gland coils, and within the connective tissue septa of the hypodermis. Dermal dendrocytes complement the immunologically functional cells of the epidermis. Mast cells are mononuclear cells of bone marrow origin, sparsely distributed in the perivascular and periadnexal dermis.
Excluding the epidermis, which is a nonvascular tissue, the skin possesses a rich vascular network that largely exceeds the skin metabolic requirements. This network plays a role in thermoregulation, wound healing, immune reactions, and blood pressure control. Cutaneous vessels belong to the arterial, venous, or lymphatic system; they originate from perforating arteries arising from underlying vessels of the muscles and form two distinct horizontal plexuses that communicate via vessels traversing the dermis vertically. The deep plexus lies close to the dermal-hypodermal junction and provides nutritional arteries to sweat glands and hair follicles. The superficial plexus, derived from terminal arterioles, lies at the interface between the papillary and reticular dermis and provides a vascular loop to every dermal papilla toward the surface (except in the nail bed). This consists of an ascending precapillary arteriole, arterial and venous capillaries forming a hairpin turn, and a descending postcapillary venule (these account for the majority of vessels in the papillary dermis).7
The skin contains a rich and complex enervation, consisting of an afferent and an efferent limb. The afferent limb is responsible for the perception of eternal stimuli (touch, pressure, vibration, pain, temperature, itch) via a network of sensory myelinated and nonmyelinated fibers, free terminal nerve endings, and tactile corpuscles. The efferent limb is supported by nonmyelinated fibers of the sympathetic system that regulate vasomotricity, sweat secretion, and piloerection.
Fatty tissue is the deepest part of the skin, separating it from the underlying muscle fascia or the periosteum. It plays an important role in thermoregulation, insulation, storage of energy, and protection from mechanical injuries.
The main cells of the hypodermis are the adipocytes—large, rounded cells with a lipid-laden cytoplasm (triglycerides, fatty acids) compressing the nucleus against the cell membrane. Adipocytes are arranged in primary and secondary lobules, the morphology of which varies according to gender and body region. These lobules are separated by connective tissue septa containing cells (fibroblasts, dendrocytes, mast cells), the deepest part of sweat glands, and vessels and nerves contributing to the formation of the corresponding dermal plexuses.
INFLAMMATORY CONDITIONS
Hidradenitis suppurativa is a chronic inflammatory disease presenting as painful subcutaneous nodules. Patients experience appreciable physical, psychological, and economical hardship and decreased quality of life when compared to patients who suffer from other chronic dermatologic disease such as psoriasis and alopecia.8 It is characterized by multiple abscesses, internetworking sinus tracts, foul-smelling exudate from draining sinuses, inflammation in the dermis, both atrophic and hypertrophic scars, ulceration, and infection, which may extend deep into the fascia. The diagnosis is made clinically without the need for imaging or laboratory tests. Affected sites are axillary, inguinal, perineal, mammary, and inframammary areas corresponding to a “milk-line” distribution.
The current pathophysiologic mechanism is that there is follicular occlusion, and not an apocrine disorder as previously believed. Hyperandrogenism does not have a proven role in the disease; poor hygiene, smoking, alcohol consumption, and bacterial involvement are thought to exacerbate rather than initiate the disease process.
Treatment varies depending on disease severity and extent. The majority of patients with early-stage disease (abscesses without significant scarring) respond to topical or systemic antibiotics (clindamycin is first-line therapy). Antiandrogens have an equivocal role in therapy. Application of various anti-inflammatory agents has been successful in limited accounts and with questionable long-term efficacy. With the goal of ablating hair follicles, radiation therapy, radiofrequency ablation, and carbon dioxide (CO2) laser ablation have been employed, again with less than satisfactory long-term results.9,10,11,12
Refractory cases respond best to wide surgical debridement of the affected sites. Recurrence rates tend to be higher in the inframammary and inguino-perineal regions, reaching up to 50%. Primary wound closure after debridement bears a high risk of recurrence and is therefore discouraged. Locoregional flaps, split-thickness skin grafting, and healing by secondary intention are other alternatives. Skin grafting has a faster healing rate compared with secondary wound closure. However, the cost of having a painful donor site and limb immobilization led most patients in one reported study to prefer secondary healing.13 Topical antimicrobial creams should be used during the healing process.
Pyoderma gangrenosum (PG) is a relatively uncommon noninfectious neutrophilic dermatosis. This disease is commonly associated with inflammatory bowel disease, rheumatoid arthritis, hematologic malignancies, and monoclonal gammopathies. Clinically, the condition is characterized by the presence of sterile pustules, which progress and ulcerate to variable depths and dimensions. The lower extremities are the most commonly affected site, although all other parts of the skin can be involved. Extracutaneous manifestations include the upper airway, eye, genitalia mucosa, lungs, spleen, and muscle. Secondary infection is common. PG is more common in women and peaks in the third to sixth decades of life. Lesion borders are purplish in color with erythematous edges. Five clinical types are identified: ulcerative, pustular, bullous, vegetative, and peristomal. Treatment of this condition is centered on treatment of the inciting disease (i.e., management of Crohn’s disease) and often includes systemic steroids or calcineurin inhibitors. Treatment of PG combines systemic, topical, and surgical modalities. Systemic therapy is instituted in widespread and progressing cases and revolves around anti-inflammatory medications. Calcineurin inhibitors (inhibitors of T-cell activation) and corticosteroids are the mainstay of therapy. Other immunomodulators include sulfa drugs (dapsone), clofazimine, thalidomide, colchicine, azathioprine, cyclophosphamide, and mycophenolate mofetil. Patients with Crohn’s disease and PG treated with infliximab (tumor necrosis factor [TNF]-α inhibitor) and etanercept (TNF-α antagonist) had a marked improvement in their PG.14,15 Once ulcers develop, topical antimicrobials should be used to decrease the likelihood of secondary infections. Wound care should be geared toward debridement of purulent exudate and devitalized tissue, while maintaining a moist environment to facilitate healing. Topical calcineurin inhibitors have been shown to be useful in peristomal PG.16 Surgical debridement should be used concomitantly with systemic therapy, as the surgical insult may trigger further PG. Wound closure can usually be achieved with split-thickness skin grafting and temporary coverage with allografts or bioengineered skin substitutes.
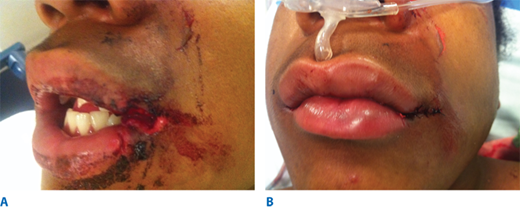
These inflammatory diseases represent a spectrum of an autoimmune reaction to stimuli such as drugs that result in structural defects in the epidermal-dermal junction. The cutaneous manifestations of toxic epidermal necrolysis syndrome (TENS) follow a prodromal period reminiscent of an upper respiratory tract infection.17 A symmetrical macular eruption follows starting from the face and trunk and spreading to the extremities. Typically, a Nikolsky sign develops in which lateral pressure causes the epidermis to detach from the basal layer. The macular eruption evolves into blisters, causing an extensive superficial partial-thickness skin injury with exposed dermis (Fig. 16-2). The process progresses for 7 to 10 days; re-epithelialization occurs over 1 to 3 weeks. Mucosal and ocular surfaces may be involved in a similar fashion. Immunosuppressed patients are at higher risk.
TENS historically was considered to be the extreme of a spectrum, with erythema multiforme and Stevens-Johnson syndrome (SJS) being less extensive forms of disease. Currently, erythema multiforme is thought to be a separate entity, related to herpetic and Mycoplasma pneumoniae infection. TENS involves more than 30% total body surface area; between 10% and 30% is considered the SJS-TEN overlap syndrome. Prognosis is related to the extent of disease and related primarily to secondary infection and other intensive care unit (ICU)-associated morbidity. With modern-day burn and ICU care, the mortality has declined significantly.17 The mildest form of the disease is SJS, which clinically presents as second-degree burns appearing as erythema and blisters/bullae of the oropharynx, anoderm, and torso. Less than 10% of total body surface area is involved with this disease. TENS is driven by the same dermo-epidermal structural defects but consists of greater than 30% total body surface area. In addition to the aforementioned, it affects the mouth, esophagus, small bowel, and colon, resulting in sloughing of mucosa that may present as gastrointestinal bleeding and intestinal malabsorption.18 It also affects the eyes, genitalia, and other mucosal surfaces.
The drugs most commonly associated with TENS-SJS include aromatic anticonvulsants, sulfonamides, allopurinol, oxicams (nonsteroidal anti-inflammatory drugs), and nevirapine. The pathophysiology of TENS is not completely understood; current theories involve apoptosis due to Fas-mediated mechanisms (a soluble or a membrane-bound protein that causes apoptosis upon activation), granulysin (a proapoptotic protein that permits cell-mediated cytotoxicity), and reactive oxygen species. There appears to be a genetic component, and genetic testing before carbamazepine treatment is recommended in people of Han Chinese ancestry to exclude carriers of HLA-B1502.19
The two principles of TENS management include early withdrawal of the offending drug and supportive care (i.e., pain control, intravenous fluid, electrolyte repletion, prevention of skin infections, enteral feeds, and possible respiratory support) in a burn unit. Despite drug withdrawal, noxious metabolites may persist. Wound care differs between centers and focuses on debridement of devitalized tissue and coverage with nonadherent dressings. Temporary skin coverage is sometimes needed until re-epithelialization is allowed to progress, reducing the probability of skin infections and dehydration. Coverage can be achieved with biologic dressing (allograft skin), biosynthetic dressings (Biobrane), and antimicrobial dressings (antibiotic or silver-impregnated such as Acticoat). A Wood’s lamp examination every 1 hour should be performed to look for corneal sloughing. It should be noted that these diseases should be distinguished from staphylococcal scalded skin syndrome, which clinically appears similar but is a result of exotoxins produced after staphylococcal infections of nasopharynx or otitis media.
Systemic treatment with steroids has fallen out of favor due to increased sepsis rates, prolonged admission, and potentially higher mortality rates. Intravenous immunoglobulin (IVIG) is thought to be a treatment given the presence of anti-Fas antibodies within IVIG. The antagonistic antibodies inhibit Fas-mediated cell apoptosis.20 However, a high variability exists between batches with this regard. There are mixed reports of IVIG treatment efficacy. A 2007 meta-analysis of nine IVIG trials concluded that high-dose IVIG does, in fact, improve survival.21 Other treatment protocols include plasmapheresis aimed at decreasing cytokine and drug load, cyclosporine, cyclophosphamide, and anti–TNF-α antibodies.17
INJURIES
Radiation-induced injuries can be the result of environmental exposure, industrial/occupational applications, and medical etiologies. Surgeons must be aware of the role radiation plays in oncologic multidisciplinary care. In addition to radiation treatment for cancers such as lymphoma and head and neck squamous cell carcinomas, radiotherapy plays a role in the adjuvant setting either before or after surgical resection in diseases such as rectal, esophageal, and cervical cancers. Although the newer modalities and principles of radiation oncology have allowed for more precise administration of this therapy with theoretically fewer side effects, there still are complications and clinical entities related to the skin (and deeper viscera) that the surgeon needs to be attuned to.
The replicating basal keratinocytes, hair follicle stem cells, and melanocytes are the most radiosensitive components of the skin. Damage to basal keratinocytes and hair follicle cells causes an immediate burst of free radicals, irreversible double-stranded breaks in nuclear and mitochondrial DNA, and inflammation. The first dose of radiation destroys a percentage of basal keratinocytes, hindering the regenerative capacity of the epidermis; repeated exposures do not allow time for cells to repair.
Acute skin changes are the result of injury to the basal epithelium in the radiated region. Within weeks, this manifests as erythema, edema, and alopecia. As the cells of the skin and subcutaneous tissue undergo repair, permanent hyperpigmentation is clinically apparent. Histologically, the epidermis appears thickened, but the functional integrity is compromised. Severe radiation injury results in complete loss of the epidermis with persistent edema and fibrinous exudate. Re-epithelialization from unaffected wound edges and from recuperating dermal adnexa begins within 10 to 14 days of exposure, provided other parameters are optimized (nutrition, infection, etc.). Chronic changes result from thrombosis and necrosis of capillaries, presenting months to years after the inciting event, ultimately leading to fibrosis and possible ulcers. Chronic skin changes include thinning, hypovascularization, telangiectasia of remaining vessels, ulceration, fibrosis with loss of elasticity, and increased susceptibility to trauma and infection. Chronic radiation skin injury includes delayed ulcers, fibrosis, and telangiectasias that present weeks to years after exposure.
Treatment of minor radiation skin injury consists primarily of maintaining the integrity of remaining skin with moisturizers until recovery of skin adnexa. Management of severe radiation includes surgical excision of damaged tissues as well as control of the typically opiate-resistant pain.
Environmental-induced injuries are from UV radiation and solar-induced skin toxicity and are the most common forms of radiation exposure skin injuries. Radiation reaching the surface of the earth contains infrared (700–2500 nm), visible (400–700 nm), and invisible UV radiation (290–400 nm).22 UVC rays are filtered by the ozone layer of the atmosphere. UVB rays (290–320 nm) and UVA rays (320–400 nm) reach the earth’s surface and have cutaneous effects. UVB radiation reaches the earth in relatively low amounts but is highly energetic. UVA rays are lower in energy, but are more abundant, constituting approximately 95% of UV rays reaching the ground. Seasonal, temporal, geo-orbital, and environmental parameters affect solar irradiance. Seventy percent of UVB radiation that reaches the skin is absorbed by the stratum corneum, 20% reaches deeper in the epidermis, and only 10% penetrates the upper part of the dermis. UVA rays are more penetrant, with 20% to 30% reaching the deep dermis. The major chromophores are nucleic acids, aromatic amino acids, and melanin.
Short-term solar radiation effects include erythema and pigmentation. UVB is more effective than UVA in causing a dermal inflammatory response resulting in erythema in a delayed phenomenon, peaking at 6 to 24 hours (dose and skin type dependent). Pigmentation occurs as a result of photo-oxidation of melanin by UVA. Partial fading occurs rapidly within 1 hour after the end of exposure. For higher UVA doses, a stable residual pigmentation is observed after the transient effect. Neomelanization is characterized by a visible brown pigmentation in UV-exposed skin, which represents an increase in epidermal melanin content. It becomes visible after about 72 hours. An acute erythemogenic dose of UVB is necessary to induce delayed pigmentation; UVA is less effective in tanning and in radiation protection. UVB pigmentation results in a homogeneous tan and UVA protection. However, melanization produced by cumulative UVA exposures appears to be longer lasting than that acquired with UVB exposures.22
Long-term effects of UV pigmentation can lead to irregular pigmentation and hyperpigmented areas, melasma, postinflammatory pigmentation, and actinic lentigines (sun spots). Radiation damage results from an increase in lysozyme activity, which inhibits the activity of collagenase and elastase and prevents the elastic fibers from proteolysis. The collagen fibril network is impaired and causes an accumulation of an amorphous, elastin-containing material. There is also a loss of collagen and a change in collagen composition (increase in collagen III to collagen I ratio). This structural disarrangement manifests as a loss of firmness and resilience of skin, leading to an older appearance to the skin.
Skin injuries may occur as a result of penetrating, blunt, and shear forces, or a combination of these. Clean lacerations may be closed primarily after irrigation, debridement, and exploration. Many surgeons will primarily close clean wounds if the injury is treated within 6 hours of the inciting event. However, there is no systematic evidence regarding the timing of closure23; practitioners are advised to use their judgment. Contaminated or infected wounds should be allowed to heal by secondary intention or delayed primary closure.24
Tangential abrasions should be approached similarly to burns injuries, with management dependent on the depth of the injury incurred. Superficial partial-thickness wounds may be left to heal spontaneously while providing topical antimicrobial prophylaxis or sterile biologic dressings. Deeper wounds may require split-thickness skin grafting to avoid prolonged need for dressing changes and hypertrophic scarring that might result from a prolonged healing period. Degloved skin may be used to provide coverage similar to a skin graft, or as a temporary dressing, provided the wound bed has been cleaned.
Accounting for over 4.5 million injuries each year, with many more presumably unreported, seemingly innocuous punctures may lead to severe deep-tissue infections if unrecognized and not treated appropriately.25
Bite bacteriology is influenced by normal mouth flora, as well as the content of the offending animal’s food. Early presentation bite wounds yield polymicrobial cultures. Common aerobic bacterial genera include Pasteurella multocida, Streptococcus, Staphylococcus, Neisseria, and Corynebacterium; anaerobic organisms include Fusobacterium, Porphyromonas, Prevotella, Propionibacterium, Bacteroides, and Peptostreptococcus. Capnocytophaga canimorsus bacteria after a dog bite are rare. It appears that immunocompromised patients are most susceptible to this type of infection and its complications. Cultures from an infected bite wound that presents late usually will grow a single organism type. Bacterial load in dog bites is heavily dependent on the content and timing of the last meal and can range between 103 with a dry meal (biscuits) to 107 within 8 hours of a wet meat meal (Fig. 16-3).26 The bacterial spectrum found in cat bites is very similar to that of dog bites, with a slightly higher prevalence of Pasteurella species. Rare infections acquired from cats have been with Francisella tularensis (tularemia) and Yersinia pestis (human plague).
Bacteria colonizing human bites are those present on the skin or in the mouth. These include the gram-positive aerobic organisms Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus species, and anaerobes including Peptococcus species, Peptostreptococcus species, Bacteroides species, and Eikenella corrodens (facultative anaerobe). Antibiotic coverage must cover gram-positive and anaerobic organisms. A first-generation cephalosporin in combination with penicillin or ampicillin in combination with clavulanic acid provides adequate coverage. Clindamycin is an alternative, but additional coverage for Eikenella corrodens should be administered.
Most wounds are amenable to standard wound care protocols. Irrigation (preferably with sterile saline solution) should be performed, although it will reduce surface bacteria only if done with nonpulsatile irrigation. To significantly reduce bacterial load and eliminate particulate debris in the wound, pressure irrigation should be performed. Eighty percent of wounds presenting to the emergency department have a bacterial load under 105, and superficial irrigation will suffice. Larger wounds with signs of infection will require pulse irrigation. Rapid quantitative cultures should be used to guide treatment in wounds with suspected infection. Human bites typically are characterized by higher bacteria load (>105).
In select cases, bite wounds may be closed primarily, particularly in areas of aesthetic significance such as the face, where secondary intention healing will result in unsightly scarring. This approach should follow initial management as outlined earlier, along with close follow-up to permit early detection of infection.
Rabies in domestic animals in the United States is rare, and the majority of cases are contracted from bat bites. In developing countries, dog bites remain the most common source of rabies. Management of this is beyond the scope of this chapter.
Between 2.4% and 10.7% of burns are due to chemical exposure27; however, approximately 30% of burn deaths are related to chemical burns. Damage from chemical burns is related to the concentration, duration, and quantity of acidic or alkaline solution. Acidic injuries typically cause a more superficial burn pattern due to eschar formation as a result of coagulation necrosis of the skin. This limits subsequent tissue penetration. Exothermic chemical reactions associated with acid burns may cause a combined thermal and chemical injury. Without treatment, this injury will result in erythema and ulcers through the subcutaneous tissue. Injuries related to basic fluids result from liquefactive necrosis starting with fat saponification and result in longer more sustained injuries causing a deeper pattern of injury (Fig. 16-4). Common examples are sodium hydroxide (drain decloggers, paint remover) and calcium hydroxide (cement). This permits further penetration of the unattached molecules, causing further tissue destruction.
The treatment for both types of injuries is based on neutralization of the inciting solution and starts with running distilled water or saline over the affected skin for at least 30 minutes for acidic solutions and 2 hours for alkaline injuries. It should be noted that neutralizing agents do not offer a significant advantage over dilution with water, may delay treatment, and may worsen the injury due to the exothermic reaction that may occur. The clinician observes and treats based on the degree of presentation. Many cases are successfully managed conservatively with topical emollients and oral analgesics, and most cases result in edema, erythema, and induration. If signs of deep second-degree burns develop, local wound care may include debridement, Silvadene, and protective petroleum gauze. In severe cases, injury to the underlying vessels, bones, muscle, and tendon may occur, and these cases may be managed within 24 hours by liposuction through a small catheter and then saline injection. Surgery is indicated for tissue necrosis, uncontrolled pain, or deep-tissue damage. Antibiotics should not be administered unless signs of infection are present.
Injuries that have specific additional treatments include hydrofluoride burns Hydrofluoride is found in air conditioning cleaners and petroleum refineries. Treatment of hydrofluoride burns should include topical or locally injected calcium gluconate to bind fluorine ions. Intra-arterial calcium gluconate can provide pain relief and preserves arteries from necrosis, whereas intravenous (IV) calcium repletes resorbed calcium stores. Topical calcium carbonate gel and quaternary ammonium compounds detoxify fluoride ions. This mitigates the leaching of calcium and magnesium ions by the hydrofluoric acid from the affected tissues and prevents potentially severe hypocalcemia and hypomagnesemia that predispose to cardiac arrhythmias.
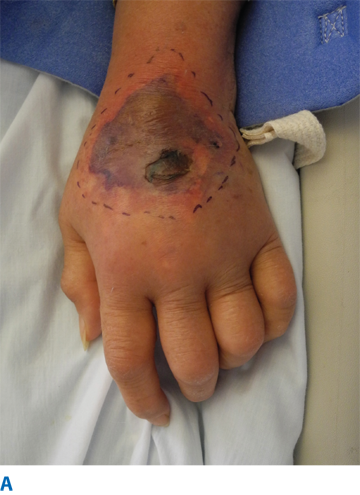
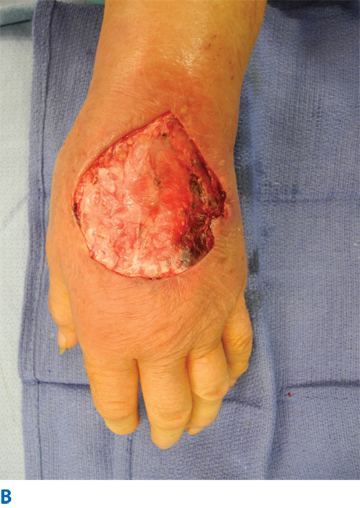
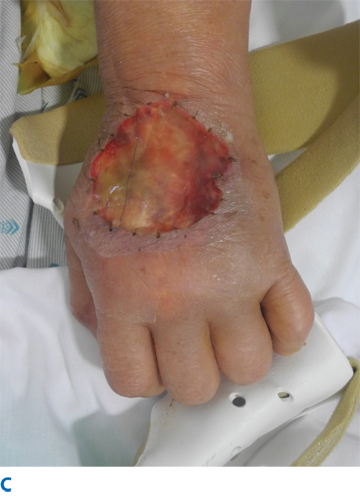
IV fluid extravasation results in yet another type of chemical injury and occurs in 0.1% to 0.7% of all cytotoxic drug administrations (Fig. 16-5). The dorsum of the hand is the most common location of this type of injury, predisposing exposure of the extensor tendon. There is a higher risk in patients receiving chemotherapy, and the risk increases dramatically inthe pediatric population (11%–58%). Doxorubicin is often the offending agent, and its effects are attributable to direct toxicity resulting in cellular death, perpetuated by release of doxorubicin from cell lysis and failed wound healing. Resultant tissue injury depends on several factors, including solution osmolality, tissue toxicity, vasoconstrictive properties, infusion pressure, and regional anatomic properties.28 Although most extravasations do not culminate in significant injuries, skin and subcutaneous damage is more likely in the critically ill and neonates. This is due, in part, to the type of infusions employed in these patients, the thin skin at the IV site (dorsum of hands and feet), fragility of veins, and the relatively poorly perfused tissue in these locations. Initial presentation may include erythema, blistering, and pain. The true extent of the injury may be beyond the apparent external margins, and this may take days to manifest completely (longer in the case of calcium carbonate infiltrations). Injury to deeper structures should be excluded. Treatment varies from conservative management with limb elevation to saline infiltration (for dilution) and aspiration with liposuction cannula.28 These methods have been shown to be effective only in the early period following extravasation. Cold or warm compresses should be avoided because they may add a thermal injury component to an area in which thermoregulatory mechanism are impeded due to vasoconstriction, pressure, and inflammation. Surgical intervention includes debridement of devitalized tissue and reconstruction with appropriate technique (i.e., skin substitutes, skin grafting, flaps, or secondary intention). Topical antimicrobial therapy is encouraged until surgery is possible.
Exposure of the skin to thermal extremes disrupts its primary function as a barrier to heat loss, evaporation, and microbial invasion. The depth and extent of injury are dependent on the duration and temperature of the exposure. The pathophysiology and management are discussed elsewhere in this book. Briefly, the epicenter of the injury undergoes a varying extent of necrosis (depending on the exposure), otherwise referred to as the zone of coagulation, which is surrounded by the zone of stasis, which has marginal perfusion and questionable viability.29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree