The Discovery of Ion Pumps
Mammalian plasma and organelle membranes are composed of phospholipids arranged in a bilayer such that the charged head groups face outward toward the water phase and the fatty acids are internal, making a hydrophobic core. For charged or hydrophilic molecules to cross the bilayer, pathways must be established to allow these molecules to move across the membrane without encountering the hydrophobic barrier. Transport proteins allow such molecules to move across the part of the protein that is placed across the hydrophobic core of the membrane, the membrane domain of the protein. In this way, these hydrophilic molecules pass across the membrane without contacting the hydrocarbon phase of the cell membranes. The ion or hydrophilic substance must be enclosed by the membrane domain of the protein; therefore, there are several chains of amino acids that surround the transported species. Transport proteins, therefore, are often rather large proteins with several amphipathic sequences of approximately twenty-one amino acids that are threaded in and out through the membrane, forming a polytopic integral membrane protein. Some of these membrane sequences are structural scaffolds, and some are responsible for the movement of molecules across the membrane. Several channel and pump proteins have been crystallized and their structure obtained by X-ray diffraction at high resolution. In this way, a description of structure-function can be generated at the atomic level.
Some transporters allow molecules to diffuse down their concentration gradient, resulting in passive transport; some couple diffusion in one direction to another molecule (usually an ion) diffusing in the opposite direction, resulting in countertransport; some couple diffusion in one direction to another molecule moving downhill in the same direction to give cotransport. There is group translocation, conversion of a diffusing substance to a different chemical on the other side of the membrane. Some, like the gastric acid pump or the sodium pump, couple energy-yielding reactions, such as the breakdown of adenosine triphosphate (ATP), directly to ion transport to produce primary active transport.
Acid secretion by the stomach was long recognized as an active transport process, because the concentration of hydrogen ions in the gastric juice is approximately 106 times higher than that in the blood. In the 1920s, the colored cytochrome oxidoreductases were discovered by Keilin and Hartree. They postulated the presence of a cytochrome chain such that electrons were transferred from a substrate, such as succinic acid, stepwise down the respiratory chain consisting of cytochrome b, then c, and then a3, eventually reaching oxygen. This chain of oxidoreductases accounted for mitochondrial oxidation of substrates. Plant physiologists then suggested that similarly oriented oxidoreductases across a membrane could separate H+ and electrons.
This concept was then applied to acid secretion by the stomach and was known as the redox hypothesis of acid secretion. Inherent in the redox hypothesis is development of a current across the membrane, providing an
electrogenic mechanism (i.e., voltage and current generating) for acid secretion across the membrane containing the pump. This electrogenic mechanism for acid secretion dominated thinking in the 1950s and early 1960s, largely due to the contributions of electrophysiologists such as Warren Rehm, first in Louisville and then in Birmingham, and Robert Davies in Sheffield. The experimental basis for this belief depended on measurements of transepithelial potential and resistance during changes in acid secretion in the in vitro frog mucosa.
electrogenic mechanism (i.e., voltage and current generating) for acid secretion across the membrane containing the pump. This electrogenic mechanism for acid secretion dominated thinking in the 1950s and early 1960s, largely due to the contributions of electrophysiologists such as Warren Rehm, first in Louisville and then in Birmingham, and Robert Davies in Sheffield. The experimental basis for this belief depended on measurements of transepithelial potential and resistance during changes in acid secretion in the in vitro frog mucosa.
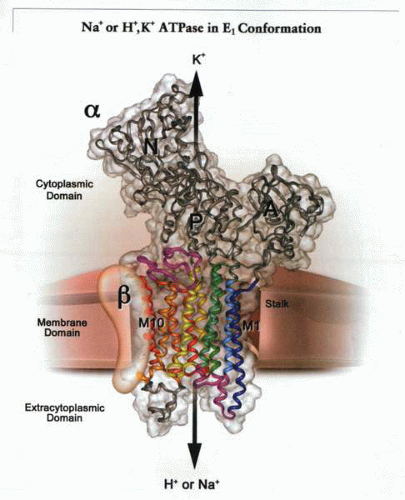 A schematic representation of a transport ATPase composed of two subunits, a catalytic or α subunit and a structural or β subunit, exchanging either intracellular Na+ or H+ for K+
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|