KEY POINTS
The breast receives its principal blood supply from perforating branches of the internal mammary artery, lateral branches of the posterior intercostal arteries, and branches from the axillary artery, including the highest thoracic, lateral thoracic, and pectoral branches of the thoracoacromial artery.
The axillary lymph nodes usually receive >75% of the lymph drainage from the breast, and the rest flows through the lymph vessels that accompany the perforating branches of the internal mammary artery and enters the parasternal (internal mammary) group of lymph nodes.
Breast development and function are initiated by a variety of hormonal stimuli, with the major trophic effects being modulated by estrogen, progesterone, and prolactin.
Benign breast disorders and diseases are related to the normal processes of reproductive life and to involution, and there is a spectrum of breast conditions that ranges from normal to disorder to disease (aberrations of normal development and involution classification).
To calculate breast cancer risk using the Gail model, a woman’s risk factors are translated into an overall risk score by multiplying her relative risks from several categories. This risk score is then compared with an adjusted population risk of breast cancer to determine the woman’s individual risk. This model is not appropriate for use in women with a known BRCA1 or BRCA2 mutation or women with lobular or ductal carcinoma in situ.
Routine use of screening mammography in women ≥50 years of age reduces mortality from breast cancer by 25%. MRI screening is recommended in women with BRCA mutations and may be considered in women with a greater than 20% to 25% lifetime risk of developing breast cancer.
Core-needle biopsy is the preferred method for diagnosis of palpable or nonpalpable breast abnormalities.
When a diagnosis of breast cancer is made, the surgeon should determine the clinical stage, histologic characteristics, and appropriate biomarker levels before initiating local therapy.
Sentinel node dissection is the preferred method for staging of the regional lymph nodes in women with clinically node-negative invasive breast cancer. Axillary dissection may be avoided in women with 1 to 2 positive sentinel nodes who are treated with breast conserving surgery, whole breast radiation and systemic therapy.
Local-regional and systemic therapy decisions for an individual patient with breast cancer are best made using a multidisciplinary treatment approach. The sequencing of therapies is dependent on patient and tumor related factors including breast cancer subtype.
A BRIEF HISTORY OF BREAST CANCER THERAPY
Breast cancer has captured the attention of surgeons throughout the ages. The Smith Surgical Papyrus (3000–2500 b.c.) is the earliest known document to refer to breast cancer. The cancer was in a man, but the description encompassed most of the common clinical features. In reference to this cancer, the author concluded, “There is no treatment.”1 There were few other historical references to breast cancer until the first century. In De Medicina, Celsus commented on the value of operations for early breast cancer: “None of these may be removed but the cacoethes (early cancer), the rest are irritated by every method of cure. The more violent the operations are, the more angry they grow.”2 In the second century, Galen inscribed his classical clinical observation: “We have often seen in the breast a tumor exactly resembling the animal the crab. Just as the crab has legs on both sides of his body, so in this disease the veins extending out from the unnatural growth take the shape of a crab’s legs. We have often cured this disease in its early stages, but after it has reached a large size, no one has cured it. In all operations we attempt to excise the tumor in a circle where it borders on the healthy tissue.”3
The galenic system of medicine ascribed cancers to an excess of black bile and concluded that excision of a local bodily outbreak could not cure the systemic imbalance. Theories espoused by Galen dominated medicine until the Renaissance. In 1652 Tulp introduced the idea that cancer was contagious when he reported an elderly woman and her housemaid who both developed breast cancer (N. Tulp, Observationes medicae 1652). This single incidence was accepted as conclusive evidence and started an idea which persisted into the 20th century among some lay people. The majority of respected surgeons considered operative intervention to be a futile and ill-advised endeavor. The Renaissance and the wars of the 16th and 17th centuries brought developments in surgery, particularly in anatomical understanding. However there were no new theories espoused in relation to cancer. Beginning with Morgagni, surgical resections were more frequently undertaken, including some early attempts at mastectomy and axillary dissection. The 17th century saw the start of the Age of Enlightenment which lasted until the 19th century. In terms of medicine, this resulted in the abandonment of Galen’s humoral pathology which was repudiated by Le Dran and the subsequent rise in cellular pathology as espoused by Virchow. Le Dran stated that breast cancer was a local disease that spread by way of lymph vessels to axillary lymph nodes. When operating on a woman with breast cancer, he routinely removed any enlarged axillary lymph nodes.4
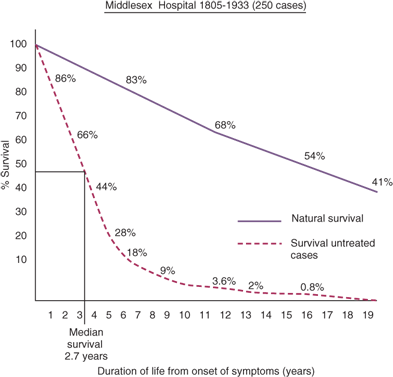
In the 19th century, Moore, of the Middlesex Hospital, London, emphasized complete resection of the breast for cancer and stated that palpable axillary lymph nodes also should be removed.5 In a presentation before the British Medical Association in 1877, Banks supported Moore’s concepts and advocated the resection of axillary lymph nodes even when palpable lymphadenopathy was not evident, recognizing that occult involvement of axillary lymph nodes was frequently present. In 1894, Halsted and Meyer reported their operations for treatment of breast cancer.6 By demonstrating superior local-regional control rates after radical resection, these surgeons established radical mastectomy as state-of-the-art treatment for that era. Halsted and Meyer advocated complete dissection of axillary lymph node levels I to III. Both routinely resected the long thoracic nerve and the thoracodorsal neurovascular bundle with the axillary contents. In 1943, Haagensen and Stout described the grave signs of breast cancer, which included: (a) edema of the skin of the breast, (b) skin ulceration, (c) chest wall fixation, (d) an axillary lymph node >2.5 cm in diameter, and (e) fixed axillary lymph nodes. Women with two or more signs had a 42% local recurrence rate and only a 2% five-year disease-free survival rate.7 Based on these findings, they declared that women with grave signs were beyond cure by radical surgery. In 1948, Patey and Dyson of the Middlesex Hospital, London, advocated a modified radical mastectomy for the management of advanced operable breast cancer, explaining, “Until an effective general agent for treatment of carcinoma of the breast is developed, a high proportion of these cases are doomed to die.”8 Their technique included removal of the breast and axillary lymph nodes with preservation of the pectoralis major muscle. They showed that removal of the pectoralis minor muscle allowed access to and clearance of axillary lymph node levels I to III.
During the 1970s, there was a transition from the Halsted radical mastectomy to the modified radical mastectomy as the surgical procedure most frequently used by American surgeons to treat breast cancer. This transition acknowledged that: (a) fewer patients were presenting with advanced local disease with or without the grave signs described by Haagensen, (b) extirpation of the pectoralis major muscle was not essential for local-regional control in stage I and II breast cancer, and (c) neither the modified radical mastectomy nor the Halsted radical mastectomy consistently achieved local-regional control of stage III breast cancer. Radiation therapy was incorporated into the management of advanced breast cancer and demonstrated improvements in local-regional control. The National Surgical Adjuvant Breast and Bowel Project (NSABP) conducted a randomized trial in the early 1970s to determine the impact of local and regional treatments on survival in operable breast cancer. In the B-04 trial, 1665 women were enrolled and stratified by clinical assessment of the axillary lymph nodes. The clinically node-negative women were randomized into three treatment groups: (a) Halsted radical mastectomy; (b) total mastectomy plus radiation therapy; and (c) total mastectomy alone. Clinically node-positive women were randomized to Halsted radical mastectomy or total mastectomy plus radiation therapy. This trial accrued patients between 1971 and 1974, an era that predated widespread availability of effective systemic therapy for breast cancer and therefore reflect survival associated with local-regional therapy alone. There were no differences in survival between the three groups of node-negative women or between the two groups of node-positive women. These overall survival equivalence patterns have persisted at 25 years of follow-up.9
The next major advance in the surgical management of breast cancer was the development of breast conserving surgery. Breast conserving surgery and radium treatment was first reported by Geoffrey Keynes of St Bartholomew’s Hospital, London in the British Medical Journal in 1937.10 Several decades later, the NSABP launched the B-06 trial, a phase III study that randomized 1851 patients to total mastectomy, lumpectomy alone, or lumpectomy with breast irradiation. The results showed no difference in disease-free, distant disease-free, and overall survival among the three groups; however, the omission of radiation therapy resulted in significantly higher rates of ipsilateral breast tumor recurrence in those who received lumpectomy alone.11 The B-06 trial excluded patients who had palpable axillary lymph nodes and those patients randomized to breast conserving surgery had frozen sections performed and if on frozen section the margins were involved the surgeon proceeded to perform a mastectomy but the patient was included in the analysis as though they had a breast conserving operation. Furthermore, in B-06 local in-breast recurrences were regarded as “non-events” in terms of disease-free survival. Both NSABP B-04 and B-06 trials were taken to refute the Halstedian concept that cancer spread throughout a region of the breast to lymphatics and then on to distant sites. Bernard Fisher proposed the “alternative hypothesis” that breast cancer was a systemic disease at diagnosis and that tumor cells had access to both the blood and lymphatic systems and that regional lymph nodes were a marker of systemic disease and not a barrier to the dissemination of cancer cells. He proposed that host factors were important in the development of metastasis and that variations in the local-regional approach to breast cancer were not likely to substantially impact survival. This idea was dominant for a number of years but has been challenged by the Early Breast Cancer Trialists’ Collaborative Group overview analysis which reported that “the avoidance of recurrence in a conserved breast …. avoids about one breast cancer death over the next 15 years for every four such recurrences avoided.”12 Indicating that not all breast cancer is a systemic disease at presentation.
During the 1970s, clinical trials were initiated to determine the value of systemic therapy in the postoperative setting as an adjuvant to surgery. The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) was established in 1985 to coordinate the meta-analysis of data from randomized clinical trials in order to examine the impact of adjuvant treatments for breast cancer on recurrence and mortality. The EBCTCG overview has demonstrated that anthracycline containing regimens are superior to CMF, and more recently, that the addition of a taxane to an anthracycline-based regimen reduces breast cancer mortality by one third.11 The overview has also demonstrated that tamoxifen is of benefit only in patients with estrogen receptor (ER) positive breast cancer and that tamoxifen may decrease mortality from breast cancer by as much as 50%.13 Importantly, the EBCTCG data have shown that proportional reduction in risk was not significantly affected by standard clinical and pathologic factors such as tumor size, ER status, and nodal status.14 This underscores the importance of stratification of risk in determining adjuvant therapy decisions in order to minimize the toxicities of therapies in those unlikely to benefit, yet realize the substantial benefits gained in local-regional control and survival in those at higher risk.
Many early randomized clinical trials considered all patients similarly in terms of treatment viewing breast cancer as more of a homogeneous disease. Breast cancer has traditionally been defined by pathologic determinants using conventional light microscopy and basic histologic techniques. In the 1980s immunohistochemistry allowed assessment of the expression of individual tumor markers (most commonly proteins) while DNA was initially assessed in terms of its ploidy status. Subsequently, breast cancer specimens have been interrogated at the level of the DNA by labeling genes of interest and allowing fluorescent dyes to quantify the abundance of a particular gene and comparing a large number of genes simultaneously in a single breast cancer specimen. Gene expression arrays have shown that breast cancers cluster according to their intrinsic gene expression patterns into at least five intrinsic subtypes and these intrinsic subtypes correlate with breast cancer outcomes.15 Breast cancers are now classified by molecular subtypes and these are being used for risk stratification and decision making in terms of local-regional and systemic therapies.
Currently, 50% of American women will consult a surgeon regarding breast disease, 25% will undergo breast biopsy for diagnosis of an abnormality, and 12% will develop some variant of breast cancer. Considerable progress has been made in the integration of surgery, radiation therapy, and systemic therapy to control local-regional disease, enhance survival, and improve the quality of life of breast cancer survivors. Surgeons are traditionally the first physician consulted for breast care and it is critical for them to be well trained in all aspects of the breast from embryologic development, to growth and development, and to benign and malignant disease processes. This will allow the greatest opportunity to achieve optimal outcomes for patients and their families.
EMBRYOLOGY AND FUNCTIONAL ANATOMY OF THE BREAST
At the fifth or sixth week of fetal development, two ventral bands of thickened ectoderm (mammary ridges, milk lines) are evident in the embryo.16 In most mammals, paired breasts develop along these ridges, which extend from the base of the forelimb (future axilla) to the region of the hind limb (inguinal area). These ridges are not prominent in the human embryo and disappear after a short time, except for small portions that may persist in the pectoral region. Accessory breasts (polymastia) or accessory nipples (polythelia) may occur along the milk line (Fig. 17-1) when normal regression fails. Each breast develops when an ingrowth of ectoderm forms a primary tissue bud in the mesenchyme. The primary bud, in turn, initiates the development of 15 to 20 secondary buds. Epithelial cords develop from the secondary buds and extend into the surrounding mesenchyme. Major (lactiferous) ducts develop, which open into a shallow mammary pit. During infancy, a proliferation of mesenchyme transforms the mammary pit into a nipple. If there is failure of a pit to elevate above skin level, an inverted nipple results. This congenital malformation occurs in 4% of infants. At birth, the breasts are identical in males and females, demonstrating only the presence of major ducts. Enlargement of the breast may be evident and a secretion, historically referred to as witch’s milk, may be produced. These transitory events occur in response to maternal hormones that cross the placenta.
The breast remains undeveloped in the female until puberty, when it enlarges in response to ovarian estrogen and progesterone, which initiate proliferation of the epithelial and connective tissue elements. However, the breasts remain incompletely developed until pregnancy occurs. Absence of the breast (amastia) is rare and results from an arrest in mammary ridge development that occurs during the sixth fetal week. Poland’s syndrome consists of hypoplasia or complete absence of the breast, costal cartilage and rib defects, hypoplasia of the subcutaneous tissues of the chest wall, and brachysyndactyly. Breast hypoplasia also may be iatrogenically induced before puberty by trauma, infection, or radiation therapy. Symmastia is a rare anomaly recognized as webbing between the breasts across the midline. Accessory nipples (polythelia) occur in <1% of infants and may be associated with abnormalities of the urinary tract (renal agenesis and cancer), abnormalities of the cardiovascular system (conduction disturbances, hypertension, congenital heart anomalies), and other conditions (pyloric stenosis, epilepsy, ear abnormalities, arthrogryposis). Supernumerary breasts may occur in any configuration along the mammary milk line but most frequently occur between the normal nipple location and the symphysis pubis. Turner’s syndrome (ovarian agenesis and dysgenesis) and Fleischer’s syndrome (displacement of the nipples and bilateral renal hypoplasia) may have polymastia as a component. Accessory axillary breast tissue is uncommon and usually is bilateral.
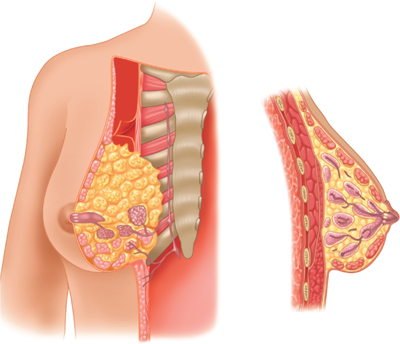
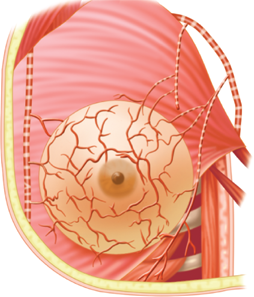
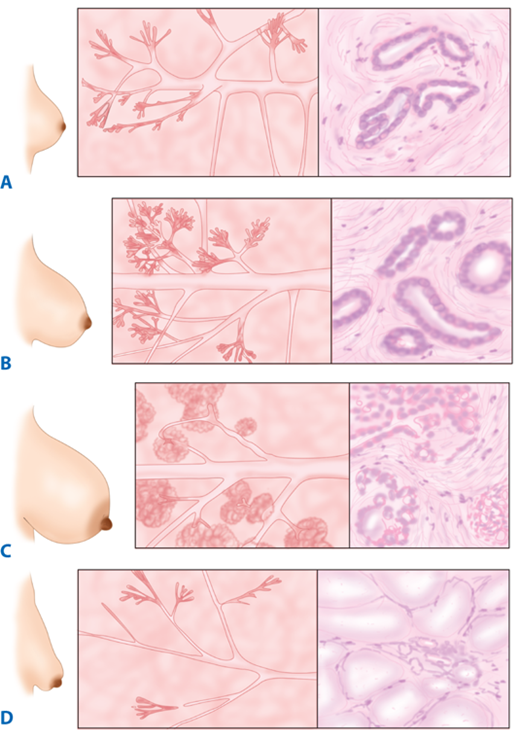
The breast is composed of 15 to 20 lobes (Fig. 17-2), which are each composed of several lobules.17 Fibrous bands of connective tissue travel through the breast (Cooper’s suspensory ligaments), insert perpendicularly into the dermis, and provide structural support. The mature female breast extends from the level of the second or third rib to the inframammary fold at the sixth or seventh rib. It extends transversely from the lateral border of the sternum to the anterior axillary line. The deep or posterior surface of the breast rests on the fascia of the pectoralis major, serratus anterior, and external oblique abdominal muscles, and the upper extent of the rectus sheath. The retromammary bursa may be identified on the posterior aspect of the breast between the investing fascia of the breast and the fascia of the pectoralis major muscles. The axillary tail of Spence extends laterally across the anterior axillary fold. The upper outer quadrant of the breast contains a greater volume of tissue than do the other quadrants. The breast has a protuberant conical form. The base of the cone is roughly circular, measuring 10 to 12 cm in diameter. Considerable variations in the size, contour, and density of the breast are evident among individuals. The nulliparous breast has a hemispheric configuration with distinct flattening above the nipple. With the hormonal stimulation that accompanies pregnancy and lactation, the breast becomes larger and increases in volume and density, whereas with senescence, it assumes a flattened, flaccid, and more pendulous configuration with decreased volume.
Figure 17-2.
Anatomy of the breast. Tangential and cross-sectional (sagittal) views of the breast and associated chest wall. (Reproduced with permission from Romrell LJ, Bland KI. Anatomy of the breast, axilla, chest wall, and related metastatic sites. In: Bland KI, Copeland EMI, eds. The Breast: Comprehensive Management of Benign and Malignant Diseases. Philadelphia: Saunders, 2009. Copyright Elsevier.)
The epidermis of the nipple-areola complex is pigmented and is variably corrugated. During puberty, the pigment becomes darker and the nipple assumes an elevated configuration. Throughout pregnancy, the areola enlarges and pigmentation is further enhanced. The areola contains sebaceous glands, sweat glands, and accessory glands, which produce small elevations on the surface of the areola (Montgomery’s tubercles). Smooth muscle bundle fibers, which lie circumferentially in the dense connective tissue and longitudinally along the major ducts, extend upward into the nipple, where they are responsible for the nipple erection that occurs with various sensory stimuli. The dermal papilla at the tip of the nipple contains numerous sensory nerve endings and Meissner’s corpuscles. This rich sensory innervation is of functional importance, because the sucking of the infant initiates a chain of neurohumoral events that results in milk letdown.
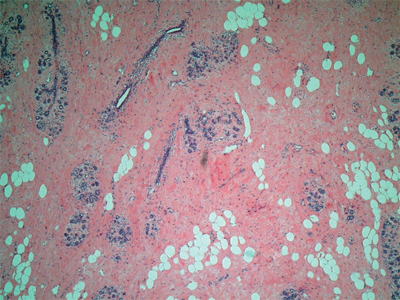
Each lobe of the breast terminates in a major (lactiferous) duct (2–4 mm in diameter), which opens through a constricted orifice (0.4–0.7 mm in diameter) into the ampulla of the nipple (see Fig. 17-2). Immediately below the nipple-areola complex, each major duct has a dilated portion (lactiferous sinus), which is lined with stratified squamous epithelium. Major ducts are lined with two layers of cuboidal cells, whereas minor ducts are lined with a single layer of columnar or cuboidal cells. Myoepithelial cells of ectodermal origin reside between the epithelial cells in the basal lamina and contain myofibrils. In the inactive breast, the epithelium is sparse and consists primarily of ductal epithelium (Fig. 17-3). In the early phase of the menstrual cycle, minor ducts are cord-like with small lumina. With estrogen stimulation at the time of ovulation, alveolar epithelium increases in height, duct lumina become more prominent, and some secretions accumulate. When the hormonal stimulation decreases, the alveolar epithelium regresses.
Figure 17-3.
Inactive human breast (100x). The epithelium, which is primarily ductal, is embedded in loose connective tissue. Dense connective tissue surrounds the terminal duct lobular units (TDLU). (Photo used with permission of Dr. Sindhu Menon, Consultant Histopathologist & Dr. Rahul Deb, Consultant Histopathologist and Lead Breast Pathologist, Royal Derby Hospital, Derby, UK.)
With pregnancy, the breast undergoes proliferative and developmental maturation. As the breast enlarges in response to hormonal stimulation, lymphocytes, plasma cells, and eosinophils accumulate within the connective tissues. The minor ducts branch and alveoli develop. Development of the alveoli is asymmetric, and variations in the degree of development may occur within a single lobule (Fig. 17-4). With parturition, enlargement of the breasts occurs via hypertrophy of alveolar epithelium and accumulation of secretory products in the lumina of the minor ducts. Alveolar epithelium contains abundant endoplasmic reticulum, large mitochondria, Golgi complexes, and dense lysosomes. Two distinct substances are produced by the alveolar epithelium: (a) the protein component of milk, which is synthesized in the endoplasmic reticulum (merocrine secretion); and (b) the lipid component of milk (apocrine secretion), which forms as free lipid droplets in the cytoplasm. Milk released in the first few days after parturition is called colostrum and has low lipid content but contains considerable quantities of antibodies. The lymphocytes and plasma cells that accumulate within the connective tissues of the breast are the source of the antibody component. With subsequent reduction in the number of these cells, the production of colostrum decreases and lipid-rich milk is released.
Figure 17-4.
Active human breast: pregnancy and lactation (160x). The alveolar epithelium becomes conspicuous during the early proliferative period. The alveolus is surrounded by cellular connective tissue. (Photo used with permission of Dr. Sindhu Menon, Consultant Histopathologist & Dr. Rahul Deb, Consultant Histopathologist and Lead Breast Pathologist, Royal Derby Hospital, Derby, UK.)
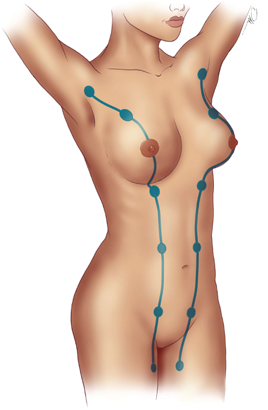
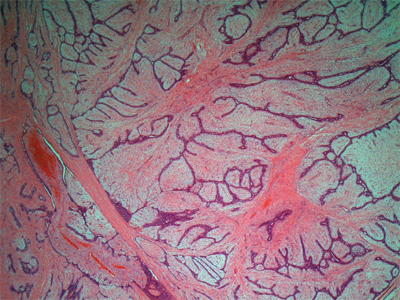
The breast receives its principal blood supply from: (a) perforating branches of the internal mammary artery; (b) lateral branches of the posterior intercostal arteries; and (c) branches from the axillary artery, including the highest thoracic, lateral thoracic, and pectoral branches of the thoracoacromial artery (Fig. 17-5). The second, third, and fourth anterior intercostal perforators and branches of the internal mammary artery arborize in the breast as the medial mammary arteries. The lateral thoracic artery gives off branches to the serratus anterior, pectoralis major and pectoralis minor, and subscapularis muscles. It also gives rise to lateral mammary branches. The veins of the breast and chest wall follow the course of the arteries, with venous drainage being toward the axilla. The three principal groups of veins are: (a) perforating branches of the internal thoracic vein, (b) perforating branches of the posterior intercostal veins, and (c) tributaries of the axillary vein. Batson’s vertebral venous plexus, which invests the vertebrae and extends from the base of the skull to the sacrum, may provide a route for breast cancer metastases to the vertebrae, skull, pelvic bones, and central nervous system. Lymph vessels generally parallel the course of blood vessels.
Figure 17-5.
Arterial supply to the breast, axilla, and chest wall. (Reproduced with permission from Romrell LJ, Bland KI. Anatomy of the breast, axilla, chest wall, and related metastatic sites. In: Bland KI, Copeland EMI, eds. The Breast: Comprehensive Management of Benign and Malignant Diseases. Philadelphia: Saunders, 2009. Copyright Elsevier.)
Lateral cutaneous branches of the third through sixth intercostal nerves provide sensory innervation of the breast (lateral mammary branches) and of the anterolateral chest wall. These branches exit the intercostal spaces between slips of the serratus anterior muscle. Cutaneous branches that arise from the cervical plexus, specifically the anterior branches of the supraclavicular nerve, supply a limited area of skin over the upper portion of the breast. The intercostobrachial nerve is the lateral cutaneous branch of the second intercostal nerve and may be visualized during surgical dissection of the axilla. Resection of the intercostobrachial nerve causes loss of sensation over the medial aspect of the upper arm.
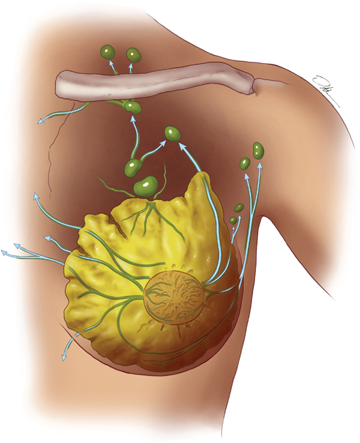
The boundaries for lymph drainage of the axilla are not well demarcated, and there is considerable variation in the position of the axillary lymph nodes. The six axillary lymph node groups recognized by surgeons (Figs. 17-6 and 17-7) are: (a) the axillary vein group (lateral), which consists of four to six lymph nodes that lie medial or posterior to the vein and receive most of the lymph drainage from the upper extremity; (b) the external mammary group (anterior or pectoral group), which consists of five to six lymph nodes that lie along the lower border of the pectoralis minor muscle contiguous with the lateral thoracic vessels and receive most of the lymph drainage from the lateral aspect of the breast; (c) the scapular group (posterior or subscapular), which consists of five to seven lymph nodes that lie along the posterior wall of the axilla at the lateral border of the scapula contiguous with the subscapular vessels and receive lymph drainage principally from the lower posterior neck, the posterior trunk, and the posterior shoulder; (d) the central group, which consists of three or four sets of lymph nodes that are embedded in the fat of the axilla lying immediately posterior to the pectoralis minor muscle and receive lymph drainage both from the axillary vein, external mammary, and scapular groups of lymph nodes, and directly from the breast; (e) the subclavicular group (apical), which consists of six to twelve sets of lymph nodes that lie posterior and superior to the upper border of the pectoralis minor muscle and receive lymph drainage from all of the other groups of axillary lymph nodes; and (f) the interpectoral group (Rotter’s lymph nodes), which consists of one to four lymph nodes that are interposed between the pectoralis major and pectoralis minor muscles and receive lymph drainage directly from the breast. The lymph fluid that passes through the interpectoral group of lymph nodes passes directly into the central and subclavicular groups.
Figure 17-7.
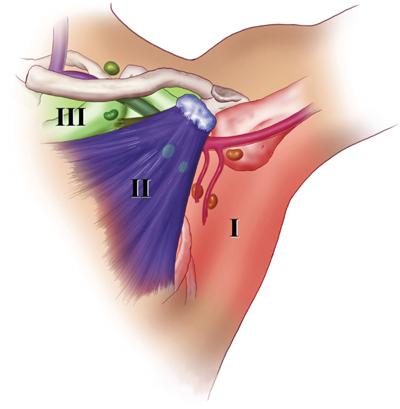
Axillary lymph node groups. Level I includes lymph nodes located lateral to the pectoralis minor muscle; level II includes lymph nodes located deep to the pectoralis minor; and level III includes lymph nodes located medial to the pectoralis minor. The axillary vein with its major tributaries and the supraclavicular lymph node group are also illustrated. (Visual Art: © 2012.The University of Texas MD Anderson Cancer Center.)
As indicated in Fig. 17-7, the lymph node groups are assigned levels according to their anatomic relationship to the pectoralis minor muscle. Lymph nodes located lateral to or below the lower border of the pectoralis minor muscle are referred to as level I lymph nodes, which include the axillary vein, external mammary, and scapular groups. Lymph nodes located superficial or deep to the pectoralis minor muscle are referred to as level II lymph nodes, which include the central and interpectoral groups. Lymph nodes located medial to or above the upper border of the pectoralis minor muscle are referred to as level III lymph nodes, which consist of the subclavicular group. The plexus of lymph vessels in the breast arises in the interlobular connective tissue and in the walls of the lactiferous ducts and communicates with the subareolar plexus of lymph vessels. Efferent lymph vessels from the breast pass around the lateral edge of the pectoralis major muscle and pierce the clavipectoral fascia, ending in the external mammary (anterior, pectoral) group of lymph nodes. Some lymph vessels may travel directly to the subscapular (posterior, scapular) group of lymph nodes. From the upper part of the breast, a few lymph vessels pass directly to the subclavicular (apical) group of lymph nodes. The axillary lymph nodes usually receive >75% of the lymph drainage from the breast. The rest is derived primarily from the medial aspect of the breast, flows through the lymph vessels that accompany the perforating branches of the internal mammary artery, and enters the parasternal (internal mammary) group of lymph nodes.
PHYSIOLOGY OF THE BREAST
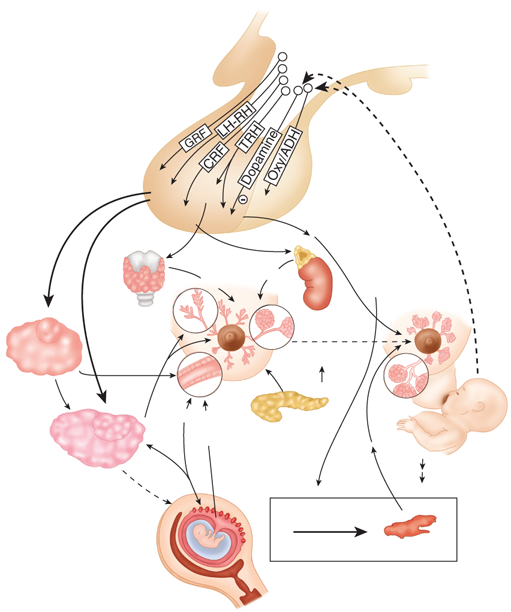
Breast development and function are initiated by a variety of hormonal stimuli, including estrogen, progesterone, prolactin, oxytocin, thyroid hormone, cortisol, and growth hormone.17,18 Estrogen, progesterone, and prolactin especially have profound trophic effects that are essential to normal breast development and function. Estrogen initiates ductal development, whereas progesterone is responsible for differentiation of epithelium and for lobular development. Prolactin is the primary hormonal stimulus for lactogenesis in late pregnancy and the postpartum period. It upregulates hormone receptors and stimulates epithelial development. Figure 17-8 depicts the secretion of neurotrophic hormones from the hypothalamus, which is responsible for regulation of the secretion of the hormones that affect the breast tissues. The gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) regulate the release of estrogen and progesterone from the ovaries. In turn, the release of LH and FSH from the basophilic cells of the anterior pituitary is regulated by the secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus. Positive and negative feedback effects of circulating estrogen and progesterone regulate the secretion of LH, FSH, and GnRH. These hormones are responsible for the development, function, and maintenance of breast tissues (Fig. 17-9A). In the female neonate, circulating estrogen and progesterone levels decrease after birth and remain low throughout childhood because of the sensitivity of the hypothalamic-pituitary axis to negative feedback from these hormones. With the onset of puberty, there is a decrease in the sensitivity of the hypothalamic-pituitary axis to negative feedback and an increase in its sensitivity to positive feedback from estrogen. These physiologic events initiate an increase in GnRH, FSH, and LH secretion and ultimately an increase in estrogen and progesterone secretion by the ovaries, leading to establishment of the menstrual cycle. At the beginning of the menstrual cycle, there is an increase in the size and density of the breasts, which is followed by engorgement of the breast tissues and epithelial proliferation. With the onset of menstruation, the breast engorgement subsides and epithelial proliferation decreases.
Figure 17-8.
Overview of the neuroendocrine control of breast development and function. ADH = antidiuretic hormone; CRF = corticotropin-releasing factor; GRF = growth hormone releasing factor; LH-RH = luteinizing hormone–releasing hormone; Oxy = oxytocin; TRH = thyrotropin-releasing hormone. (Reproduced with permission from Kass R et al. Breast physiology: normal and abnormal development and function. In: Bland KI, Copeland EMI, eds. The Breast: Comprehensive Management of Benign and Malignant Diseases. Philadelphia: Saunders, 2009. Copyright Elsevier.)
A dramatic increase in circulating ovarian and placental estrogens and progestins is evident during pregnancy, which initiates striking alterations in the form and substance of the breast (see Fig. 17-9B).17,18,19 The breast enlarges as the ductal and lobular epithelium proliferates, the areolar skin darkens, and the accessory areolar glands (Montgomery’s glands) become prominent. In the first and second trimesters, the minor ducts branch and develop. During the third trimester, fat droplets accumulate in the alveolar epithelium and colostrum fills the alveolar and ductal spaces. In late pregnancy, prolactin stimulates the synthesis of milk fats and proteins.
After delivery of the placenta, circulating progesterone and estrogen levels decrease, permitting full expression of the lactogenic action of prolactin. Milk production and release are controlled by neural reflex arcs that originate in nerve endings of the nipple-areola complex. Maintenance of lactation requires regular stimulation of these neural reflexes, which results in prolactin secretion and milk letdown. Oxytocin release results from the auditory, visual, and olfactory stimuli associated with nursing. Oxytocin initiates contraction of the myoepithelial cells, which results in compression of alveoli and expulsion of milk into the lactiferous sinuses. After weaning of the infant, prolactin and oxytocin release decreases. Dormant milk causes increased pressure within the ducts and alveoli, which results in atrophy of the epithelium (Fig. 17-9C). With menopause there is a decrease in the secretion of estrogen and progesterone by the ovaries and involution of the ducts and alveoli of the breast. The surrounding fibrous connective tissue increases in density, and breast tissues are replaced by adipose tissues (Fig. 17-9D).
Gynecomastia refers to an enlarged breast in the male.20 Physiologic gynecomastia usually occurs during three phases of life: the neonatal period, adolescence, and senescence. Common to each of these phases is an excess of circulating estrogens in relation to circulating testosterone. Neonatal gynecomastia is caused by the action of placental estrogens on neonatal breast tissues, whereas in adolescence, there is an excess of estradiol relative to testosterone, and with senescence, the circulating testosterone level falls, which results in relative hyperestrinism. In gynecomastia, the ductal structures of the male breast enlarge, elongate, and branch with a concomitant increase in epithelium. During puberty, the condition often is unilateral and typically occurs between ages 12 and 15 years. In contrast, senescent gynecomastia is usually bilateral. In the nonobese male, breast tissue measuring at least 2 cm in diameter must be present before a diagnosis of gynecomastia may be made. Mammography and ultrasonography are used to differentiate breast tissues. Dominant masses or areas of firmness, irregularity, and asymmetry suggest the possibility of a breast cancer, particularly in the older male. Gynecomastia generally does not predispose the male breast to cancer. However, the hypoandrogenic state of Klinefelter’s syndrome (XXY), in which gynecomastia is usually evident, is associated with an increased risk of breast cancer. Gynecomastia is graded based on the degree of breast enlargement, the position of the nipple with reference to the inframammary fold and the degree of breast ptosis and skin redundancy: Grade 1: mild breast enlargement without skin redundancy; Grade IIa: moderate breast enlargement without skin redundancy; Grade IIb: moderate breast enlargement with skin redundancy; and Grade 3: marked breast enlargement with skin redundancy and ptosis.
Table 17-1 identifies the pathophysiologic mechanisms that may initiate gynecomastia: estrogen excess states; androgen deficiency states; pharmacologic causes; and idiopathic causes. Estrogen excess results from an increase in the secretion of estradiol by the testicles or by nontesticular tumors, nutritional alterations such as protein and fat deprivation, endocrine disorders (hyperthyroidism, hypothyroidism), and hepatic disease (nonalcoholic and alcoholic cirrhosis). Refeeding gynecomastia is related to the resumption of pituitary gonadotropin secretion after pituitary shutdown. Androgen deficiency may initiate gynecomastia. Concurrently occurring with decreased circulating testosterone levels is an elevated level of circulating testosterone-binding globulin, which results in a reduction of free testosterone. This senescent gynecomastia usually occurs in men aged 50 to 70 years. Hypoandrogenic states can be from primary testicular failure or secondary testicular failure. Klinefelter’s syndrome (XXY) is an example of primary testicular failure which is manifested by gynecomastia, hypergonadotropic hypogonadism, and azoospermia. Secondary testicular failure may result from trauma, orchitis, and cryptorchidism. Renal failure, regardless of cause, also may initiate gynecomastia.
I. Estrogen excess states A. Gonadal origin 1. True hermaphroditism 2. Gonadal stromal (nongerminal) neoplasms of the testis a. Leydig cell (interstitial) b. Sertoli cell c. Granulosa-theca cell 3. Germ cell tumors a. Choriocarcinoma b. Seminoma, teratoma c. Embryonal carcinoma B. Nontesticular tumors 1. Adrenal cortical neoplasms 2. Lung carcinoma 3. Hepatocellular carcinoma C. Endocrine disorders D. Diseases of the liver—nonalcoholic and alcoholic cirrhosis E. Nutrition alteration states II. Androgen deficiency states A. Senescence B. Hypoandrogenic states (hypogonadism) 1. Primary testicular failure a. Klinefelter’s syndrome (XXY) b. Reifenstein’s syndrome c. Rosewater-Gwinup-Hamwi familial gynecomastia d. Kallmann syndrome e. Kennedy’s disease with associated gynecomastia f. Eunuchoidal state (congenital anorchia) g. Hereditary defects of androgen biosynthesis h. Adrenocorticotropic hormone deficiency 2. Secondary testicular failure a. Trauma b. Orchitis c. Cryptorchidism d. Irradiation C. Renal failure III. Pharmacologic causes IV. Systemic diseases with idiopathic mechanisms |
Pharmacologic causes of gynecomastia include drugs with estrogenic activity (digitalis, estrogens, anabolic steroids, marijuana) or drugs that enhance estrogen synthesis (human chorionic gonadotropin). Drugs that inhibit the action or synthesis of testosterone (cimetidine, ketoconazole, phenytoin, spironolactone, antineoplastic agents, diazepam) also have been implicated. Drugs such as reserpine, theophylline, verapamil, tricyclic antidepressants, and furosemide induce gynecomastia through idiopathic mechanisms.
When gynecomastia is caused by androgen deficiency, then testosterone administration may cause regression. When it is caused by medications, then these are discontinued if possible. When endocrine defects are responsible, then these receive specific therapy. As soon asgynecomastia is progressive and does not respond to other treatments, surgical therapy is considered. Techniques include local excision, liposuction or subcutaneous mastectomy. Attempts to reverse gynecomastia with danazol have been successful, but the androgenic side effects of the drug are considerable.
INFECTIOUS AND INFLAMMATORY DISORDERS OF THE BREAST
Infections in the postpartum period remain proportionately the most common time for breast infections to occur. Infections of the breast unrelated to lactation are proportionately less common, however, are still a relatively common presentation to breast specialists. The latter are classified as intrinsic (secondary to abnormalities in the breast) or extrinsic (secondary to an infection in an adjacent structure, e.g., skin, thoracic cavity) the most common being probably periductal mastitis and infected sebaceous cyst, respectively.
Staphylococcus aureus and Streptococcus species are the organisms most frequently recovered from nipple discharge from an infected breast.17 Typically breast abscesses are seen in staphylococcal infections and present with point tenderness, erythema, and hyperthermia. When these abscesses are related to lactation they usually occur within the first few weeks of breastfeeding. If there is progression of a staphylococcal infection, this may result in subcutaneous, subareolar, interlobular (periductal), and retromammary abscesses (unicentric or multicentric). Previously almost all breast abscesses were treated by operative incision and drainage but now the initial approach is antibiotics and repeated aspiration of the abscess, usually ultrasound guided aspiration.21 Operative drainage is now reserved for those cases which don’t resolve with repeated aspiration and antibiotic therapy or if there is some other indication for incision and drainage (e.g., thinning or necrosis of the overlying skin). Preoperative ultrasonography is effective in delineating the required extent of the drainage procedure. While staphylococcal infections tend to be more localized and may be situated deep in the breast tissues, streptococcal infections usually present with diffuse superficial involvement. They are treated with local wound care, including application of warm compresses, and the administration of IV antibiotics (penicillins or cephalosporins). Breast infections may be chronic, possibly with recurrent abscess formation. In this situation, cultures are performed to identify acid-fast bacilli, anaerobic and aerobic bacteria, and fungi. Uncommon organisms may be encountered, and long-term antibiotic therapy may be required.
Biopsy of the abscess cavity wall should be considered at the time of incision and drainage to rule out underlying breast cancer in patients where antibiotics and drainage have been ineffective.
Nowadays hospital-acquired puerperal infections of the breast are much less common, but nursing women who present with milk stasis or noninfectious inflammation may still develop this problem. Epidemic puerperal mastitis is initiated by highly virulent strains of methicillin-resistant S. aureus that are transmitted via the suckling neonate and may result in substantial morbidity and occasional mortality. Purulent fluid may be expressed from the nipple. In this circumstance, breastfeeding is stopped, antibiotics are started, and surgical therapy is initiated. Nonepidemic (sporadic) puerperal mastitis refers to involvement of the interlobular connective tissue of the breast by an infectious process. The patient develops nipple fissuring and milk stasis, which initiates a retrograde bacterial infection. Emptying of the breast using breast suction pumps shortens the duration of symptoms and reduces the incidence of recurrences. The addition of antibiotic therapy results in a satisfactory outcome in >95% of cases.
Zuska’s disease, also called recurrent periductal mastitis, is a condition of recurrent retroareolar infections and abscesses.22,23 Smoking has been implicated as a risk factor for this condition.24,25 This syndrome is managed symptomatically by antibiotics coupled with incision and drainage as necessary. Attempts to obtain durable long-term control by wide débridement of chronically infected tissue and/or terminal duct resection have been reported and can be curative but equally can be frustrated by postoperative infections.26
Fungal infections of the breast are rare and usually involve blastomycosis or sporotrichosis.27 Intraoral fungi that are inoculated into the breast tissue by the suckling infant initiate these infections, which present as mammary abscesses in close proximity to the nipple-areola complex. Pus mixed with blood may be expressed from sinus tracts. Antifungal agents can be administered for the treatment of systemic (noncutaneous) infections. This therapy generally eliminates the necessity of surgical intervention, but occasionally drainage of an abscess, or even partial mastectomy, may be necessary to eradicate a persistent fungal infection. Candida albicans affecting the skin of the breast presents as erythematous, scaly lesions of the inframammary or axillary folds. Scrapings from the lesions demonstrate fungal elements (filaments and binding cells). Therapy involves the removal of predisposing factors such as maceration and the topical application of nystatin.
Hidradenitis suppurativa of the nipple-areola complex or axilla is a chronic inflammatory condition that originates within the accessory areolar glands of Montgomery or within the axillary sebaceous glands.27 Women with chronic acne are predisposed to developing hidradenitis. When located in and about the nipple-areola complex, this disease may mimic other chronic inflammatory states, Paget’s disease of the nipple, or invasive breast cancer. Involvement of the axillary skin is often multifocal and contiguous. Antibiotic therapy with incision and drainage of fluctuant areas is appropriate treatment. Excision of the involved areas may be required. Large areas of skin loss may necessitate coverage with advancement flaps or split-thickness skin grafts.
Mondor’s disease is a variant of thrombophlebitis that involves the superficial veins of the anterior chest wall and breast.28 In 1939, Mondor described the condition as “string phlebitis,” a thrombosed vein presenting as a tender, cord-like structure.29 Frequently involved veins include the lateral thoracic vein, the thoracoepigastric vein, and, less commonly, the superficial epigastric vein. Typically, a woman presents with acute pain in the lateral aspect of the breast or the anterior chest wall. A tender, firm cord is found to follow the distribution of one of the major superficial veins. Rarely, the presentation is bilateral, and most women have no evidence of thrombophlebitis in other anatomic sites. This benign, self-limited disorder is not indicative of a cancer. When the diagnosis is uncertain, or when a mass is present near the tender cord, biopsy is indicated. Therapy for Mondor’s disease includes the liberal use of anti-inflammatory medications and application of warm compresses along the symptomatic vein. The process usually resolves within 4 to 6 weeks. When symptoms persist or are refractory to therapy, excision of the involved vein segment may be considered.
COMMON BENIGN DISORDERS AND DISEASES OF THE BREAST
Benign breast disorders and diseases encompass a wide range of clinical and pathologic entities. Surgeons require an in-depth understanding of benign breast disorders and diseases so that clear explanations may be given to affected women, appropriate treatment instituted, and unnecessary long-term follow up avoided.
The basic principles underlying the aberrations of normal development and involution (ANDI) classification of benign breast conditions are the following: (a) benign breast disorders and diseases are related to the normal processes of reproductive life and to involution; (b) there is a spectrum of breast conditions that ranges from normal to disorder to disease; and (c) the ANDI classification encompasses all aspects of the breast condition, including pathogenesis and the degree of abnormality .30 The horizontal component of Table 17-2 defines ANDI along a spectrum from normal, to mild abnormality (disorder), to severe abnormality (disease). The vertical component indicates the period during which the condition develops.
NORMAL | DISORDER | DISEASE | |
|---|---|---|---|
Early reproductive years (age 15–25 y) | Lobular development | Fibroadenoma | Giant fibroadenoma |
Stromal development | Adolescent hypertrophy | Gigantomastia | |
Nipple eversion | Nipple inversion | Subareolar abscess | |
Mammary duct fistula | |||
Later reproductive years (age 25–40 y) | Cyclical changes of menstruation | Cyclical mastalgia | Incapacitating mastalgia |
Nodularity | |||
Epithelial hyperplasia of pregnancy | Bloody nipple discharge | ||
Involution (age 35–55 y) | Lobular involution | Macrocysts | — |
Sclerosing lesions | |||
Duct involution | |||
Dilatation | Duct ectasia | Periductal mastitis | |
Sclerosis | Nipple retraction | — | |
Epithelial turnover | Epithelial hyperplasia | Epithelial hyperplasia with atypia |
Fibroadenomas are seen and present symptomatically predominantly in younger women aged 15 to 25 years (Fig. 17-10).31 Fibroadenomas usually grow to 1 or 2 cm in diameter and then are stable but may grow to a larger size. Small fibroadenomas (≤1 cm in size) are considered normal, whereas larger fibroadenomas (≤3 cm) are disorders and giant fibroadenomas (>3 cm) are disease. Similarly, multiple fibroadenomas (more than five lesions in one breast) are very uncommon and are considered disease. It is noted that with the introduction of mammographic screening, asymptomatic fibroadenomas are sometimes found in an older screened population. The precise etiology of adolescent breast hypertrophy is unknown. A spectrum of changes from limited to massive stromal hyperplasia (gigantomastia) is seen. Nipple inversion is a disorder of development of the major ducts, which prevents normal protrusion of the nipple. Mammary duct fistulas arise when nipple inversion predisposes to major duct obstruction, leading to recurrent subareolar abscess and mammary duct fistula.
Figure 17-10.
Fibroadenoma (40x). These benign tumors are typically well circumscribed and are comprised of both stromal and glandular elements. (Photo used with permission of Dr. Sindhu Menon, Consultant Histopathologist & Dr. Rahul Deb, Consultant Histopathologist and Lead Breast Pathologist, Royal Derby Hospital, Derby, UK.)
Cyclical mastalgia and nodularity usually are associated with premenstrual enlargement of the breast and are regarded as normal. Cyclical pronounced mastalgia and severe painful nodularity are viewed differently than are physiologic discomfort and lumpiness. Painful nodularity that persists for >1 week of the menstrual cycle is considered a disorder. In epithelial hyperplasia of pregnancy, papillary projections sometimes give rise to bilateral bloody nipple discharge.
Involution of lobular epithelium is dependent on the specialized stroma around it. However, an integrated involution of breast stroma and epithelium is not always seen, and disorders of the process are common. When the stroma involutes too quickly, alveoli remain and form microcysts, which are precursors of macrocysts. The macrocysts are common, often subclinical, and do not require specific treatment. Sclerosing adenosis is considered a disorder of both the proliferative and the involutional phases of the breast cycle. Duct ectasia (dilated ducts) and periductal mastitis are other important components of the ANDI classification. Periductal fibrosis is a sequela of periductal mastitis and may result in nipple retraction. About 60% of women ≥70 years of age exhibit some degree of epithelial hyperplasia (Fig. 17-11). Atypical proliferative diseases include ductal and lobular hyperplasia, both of which display some features of carcinoma in situ. Women with atypical ductal or lobular hyperplasia have a fourfold increase in breast cancer risk (Table 17-3).
Figure 17-11.
A. Ductal epithelial hyperplasia. The irregular intracellular spaces and variable cell nuclei distinguish this process from carcinoma in situ. B. Lobular hyperplasia. The presence of alveolar lumina and incomplete distention distinguish this process from carcinoma in situ. (Photos used with permission of Dr. R.L. Hackett.)
ABNORMALITY | RELATIVE RISK |
|---|---|
Nonproliferative lesions of the breast | No increased risk |
Sclerosing adenosis | No increased risk |
Intraductal papilloma | No increased risk |
Florid hyperplasia | 1.5 to 2-fold |
Atypical lobular hyperplasia | 4-fold |
Atypical ductal hyperplasia | 4-fold |
Ductal involvement by cells of atypical ductal hyperplasia | 7-fold |
Lobular carcinoma in situ | 10-fold |
Ductal carcinoma in situ | 10-fold |
Of paramount importance for the optimal management of benign breast disorders and diseases is the histologic differentiation of benign, atypical, and malignant changes.32,33 Determining the clinical significance of these changes is a problem that is compounded by inconsistent nomenclature. The classification system originally developed by Page separates the various types of benign breast disorders and diseases into three clinically relevant groups: nonproliferative disorders, proliferative disorders without atypia, and proliferative disorders with atypia (Table 17-4). Nonproliferative disorders of the breast account for 70% of benign breast conditions and carry no increased risk for the development of breast cancer. This category includes cysts, duct ectasia, periductal mastitis, calcifications, fibroadenomas, and related disorders.
Nonproliferative disorders of the breast Cysts and apocrine metaplasia Duct ectasia Mild ductal epithelial hyperplasia Calcifications Fibroadenoma and related lesions Proliferative breast disorders without atypia Sclerosing adenosis Radial and complex sclerosing lesions Ductal epithelial hyperplasia Intraductal papillomas Atypical proliferative lesions Atypical lobular hyperplasia Atypical ductal hyperplasia |
Breast macrocysts are an involutional disorder, have a high frequency of occurrence, and are often multiple. Duct ectasia is a clinical syndrome characterized by dilated subareolar ducts that are palpable and often associated with thick nipple discharge. Haagensen regarded duct ectasia as a primary event that led to stagnation of secretions, epithelial ulceration, and leakage of duct secretions (containing chemically irritating fatty acids) into periductal tissue.34 This sequence was thought to produce a local inflammatory process with periductal fibrosis and subsequent nipple retraction. An alternative theory considers periductal mastitis to be the primary process, which leads to weakening of the ducts and secondary dilatation. It is possible that both processes occur and together explain the wide spectrum of problems seen, which include nipple discharge, nipple retraction, inflammatory masses, and abscesses.
Calcium deposits are frequently encountered in the breast. Most are benign and are caused by cellular secretions and debris or by trauma and inflammation. Calcifications that are associated with cancer include microcalcifications, which vary in shape and density and are <0.5 mm in size, and fine, linear calcifications, which may show branching. Fibroadenomas have abundant stroma with histologically normal cellular elements. They show hormonal dependence similar to that of normal breast lobules in that they lactate during pregnancy and involute in the postmenopausal period. Adenomas of the breast are well circumscribed and are composed of benign epithelium with sparse stroma, which is the histologic feature that differentiates them from fibroadenomas. They may be divided into tubular adenomas and lactating adenomas. Tubular adenomas are seen in young nonpregnant women, whereas lactating adenomas are seen during pregnancy or during the postpartum period. Hamartomas are discrete breast tumors that are usually 2 to 4 cm in diameter, firm, and sharply circumscribed. Adenolipomas consist of sharply circumscribed nodules of fatty tissue that contain normal breast lobules and ducts.
The term fibrocystic disease is nonspecific. Too frequently, it is used as a diagnostic term to describe symptoms, to rationalize the need for breast biopsy, and to explain biopsy results. Synonyms include fibrocystic changes, cystic mastopathy, chronic cystic disease, chronic cystic mastitis, Schimmelbusch’s disease, mazoplasia, Cooper’s disease, Reclus’ disease, and fibroadenomatosis. Fibrocystic disease refers to a spectrum of histopathologic changes that are best diagnosed and treated specifically.
Pathology of Proliferative Disorders Without Atypia
Proliferative breast disorders without atypia include sclerosing adenosis, radial scars, complex sclerosing lesions, ductal epithelial hyperplasia, and intraductal papillomas.32,33 Sclerosing adenosis is prevalent during the childbearing and perimenopausal years and has no malignant potential. Histologic changes are both proliferative (ductal proliferation) and involutional (stromal fibrosis, epithelial regression). Sclerosing adenosis is characterized by distorted breast lobules and usually occurs in the context of multiple microcysts, but occasionally presents as a palpable mass. Benign calcifications are often associated with this disorder. Sclerosing adenosis can be managed by observation as long as the imaging features and pathologic findings are concordant. Central sclerosis and various degrees of epithelial proliferation, apocrine metaplasia, and papilloma formation characterize radial scars and complex sclerosing lesions of the breast. Lesions up to 1 cm in diameter are called radial scars, whereas larger lesions are called complex sclerosing lesions. Radial scars originate at sites of terminal duct branching where the characteristic histologic changes radiate from a central area of fibrosis. All of the histologic features of a radial scar are seen in the larger complex sclerosing lesions, but there is a greater disturbance of structure with papilloma formation, apocrine metaplasia, and occasionally sclerosing adenosis. Distinguishing between a radial scar and invasive breast carcinoma can be challenging based on core needle biopsy sampling. Often the imaging features of a radial scar (which can be quite similar to an invasive cancer) will dictate the need for either a vacuum assisted biopsy or surgical excision in order to exclude the possibility of carcinoma.
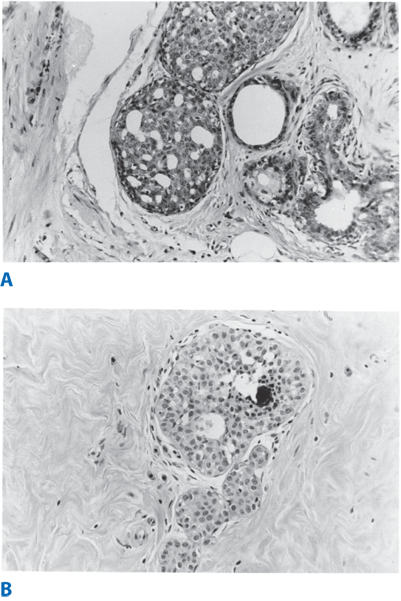
Mild ductal hyperplasia is characterized by the presence of three or four cell layers above the basement membrane. Moderate ductal hyperplasia is characterized by the presence of five or more cell layers above the basement membrane. Florid ductal epithelial hyperplasia occupies at least 70% of a minor duct lumen. It is found in >20% of breast tissue specimens, is either solid or papillary, and is associated with an increased cancer risk (see Table 17-3). Intraductal papillomas arise in the major ducts, usually in premenopausal women. They generally are <0.5 cm in diameter but may be as large as 5 cm. A common presenting symptom is nipple discharge, which may be serous or bloody. Grossly, intraductal papillomas are pinkish tan, friable, and usually attached to the wall of the involved duct by a stalk. They rarely undergo malignant transformation, and their presence does not increase a woman’s risk of developing breast cancer (unless accompanied by atypia). However, multiple intraductal papillomas, which occur in younger women and are less frequently associated with nipple discharge, are susceptible to malignant transformation.
The atypical proliferative diseases have some of the features of carcinoma in situ but either lack a major defining feature of carcinoma in situ or have the features in less than fully developed form.34 Atypical ductal hyperplasia (ADH) appears similar to low grade ductal carcinoma in situ (DCIS) histologically and is composed of monotonous round, cuboidal, or polygonal cells enclosed by basement membrane with rare mitoses. A lesion will be considered to be ADH if it is up to 2 or 3 mm in size but would be called DCIS if it is larger than 3 mm. The diagnosis can be difficult to establish with core needle biopsy specimen alone and most cases will require excisional biopsy specimen for classification. Individuals with a diagnosis of ADH are at increased risk for development of breast cancer and should be counseled appropriately regarding risk reduction strategies.
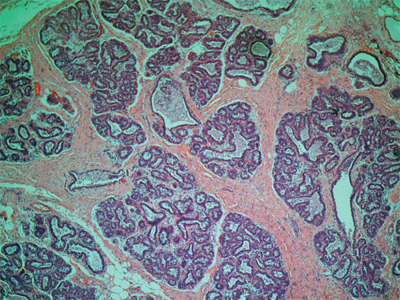
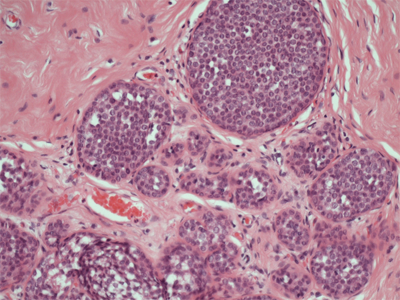
In 1978, Haagensen et al described lobular neoplasia, a spectrum of disorders ranging from atypical lobular hyperplasia to lobular carcinoma in situ (LCIS).35Atypical lobular hyperplasia (ALH) results in minimal distention of lobular units with cells that are similar to those seen in LCIS. The diagnosis of LCIS is made when small monomorphic cells that distend the terminal ductal lobular unit are noted. In cases of LCIS the acini are full and distended while the overall lobular architecture is maintained (Fig. 17-12). Classic LCIS is not associated with a specific mammographic or palpable abnormality but is an incidental finding noted on breast biopsy. There is a variant of LCIS that has been termed pleomorphic LCIS. In the case of pleomorphic LCIS, there can be calcifications or other suspicious mammographic changes that dictate the need for biopsy. Classic LCIS is not treated with excision as the patient is at risk for developing invasive breast cancer in either breast and therefore the patient is counseled regarding appropriate risk reduction strategies. Pleomorphic LCIS can be difficult to distinguish from high-grade DCIS and there are some proponents who have suggested that patients with pleomorphic LCIS be managed similar to those with DCIS with attention to margins and consideration for radiation therapy in the setting of breast conserving treatment. The use of immunohistochemical staining for E-cadherin can help to discriminate between LCIS and DCIS. In lobular neoplasias, such as ALH and LCIS, there is a lack of E-cadherin expression whereas the majority of ductal lesions will demonstrate E-cadherin reactivity.
Figure 17-12.
Lobular carcinoma in situ (100x). There are small monomorphic cells which distend the terminal duct lobular unit, without necrosis or mitoses. (Photo used with permission of Dr. Sindhu Menon, Consultant Histopathologist & Dr. Rahul Deb, Consultant Histopathologist and Lead Breast Pathologist, Royal Derby Hospital, Derby, UK.)
Because needle biopsy of breast masses may produce artifacts that make mammography assessment more difficult, many multidisciplinary teams prefer to image breast masses before performing either fine needle aspiration or core needle biopsy.36,37 In practice, however, the first investigation of palpable breast masses may be a needle biopsy, which allows for the early diagnosis of cysts. A 21-gauge needle attached to a 10-mL syringe is placed directly into the mass, which is fixed by fingers of the nondominant hand. The volume of a typical cyst is 5 to 10 mL, but it may be 75 mL or more. If the fluid that is aspirated is not bloodstained, then the cyst is aspirated to dryness, the needle is removed, and the fluid is discarded, because cytologic examination of such fluid is not cost effective. After aspiration, the breast is carefully palpated to exclude a residual mass. In most cases however imaging has been performed prior to a needle being introduced into the breast and indeed the majority of cysts are now aspirated under ultrasound guidance. If a mass was noted on initial ultrasound or there is a residual mass post-aspiration then a tissue specimen is obtained usually by core biopsy. When cystic fluid is bloodstained, fluid can be sent for cytologic examination. A simple cyst is rarely of concern, but a complex cyst may be the result of an underlying malignancy. A pneumocystogram can be obtained by injecting air into the cyst and then obtaining a repeat mammogram. When this technique is used, the wall of the cyst cavity can be more carefully assessed for any irregularities.
Most fibroadenomas are self-limiting and many go undiagnosed, so a more conservative approach is reasonable. Careful ultrasound examination with core-needle biopsy will provide for an accurate diagnosis. Ultrasonography may reveal specific features that are pathognomonic for fibroadenoma and in a young woman (e.g., under 25 years) where the risk of breast cancer is already very low a core-needle biopsy may not be necessary. In patients where biopsy is performed, the patient is counseled concerning the ultrasound and biopsy results, and surgical excision of the fibroadenoma may be avoided. Cryoablation and ultrasound-guided vacuum assisted biopsy are approved treatments for fibroadenomas of the breast, especially lesions <3 cm. Larger lesions are often still best removed by excision. With short-term follow-up a significant percentage of fibroadenomas will decrease in size and will no longer be palpable.38 However, many will remain palpable, especially those larger than 2 cm.39 Therefore, women should be counseled that the options for treatment include surgical removal, cryoablation, vacuum assisted biopsy, or observation.
The clinical significance of sclerosing adenosis lies in its imitation of cancer. On physical examination, it may be confused with cancer, by mammography, and at gross pathologic examination. Excisional biopsy and histologic examination are frequently necessary to exclude the diagnosis of cancer. The diagnostic work-up for radial scars and complex sclerosing lesions frequently involves stereotactic biopsy. It usually is not possible to differentiate these lesions with certainty from cancer by mammographic features, so a larger tissue biopsy is recommended either by way of vacuum assisted biopsy or an open surgical excisional biopsy. The mammographic appearance of a radial scar or sclerosing adenosis (mass density with spiculated margins) will usually lead to an assessment that the results of a core-needle biopsy specimen showing benign disease are discordant with the radiographic findings.
Painful and tender masses behind the nipple-areola complex are aspirated with a 21-gauge needle attached to a 10-mL syringe. Any fluid obtained is submitted for culture using a transport medium appropriate for the detection of anaerobic organisms. In the absence of pus, women are started on a combination of metronidazole and dicloxacillin while awaiting the results of culture. Antibiotics are then continued based on sensitivity tests. Many cases respond satisfactorily to antibiotics alone, but when considerable purulent material is present, repeated ultrasound guided aspiration is performed and ultimately in a proportion of cases surgical treatment is required. Unlike puerperal abscesses, a subareolar abscess is usually unilocular and often is associated with a single duct system. Ultrasound will accurately delineate its extent. In those cases which come to surgery, the surgeon may either undertake simple drainage with a view toward formal surgery, should the problem recur, or proceed with definitive surgery. In a woman of childbearing age, simple drainage is preferred, but if there is an anaerobic infection, recurrent infection frequently develops. Recurrent abscess with fistula is a difficult problem. Treatment of periductal fistula was initially recommended to be opening up of the fistulous track and allowing it to granulate.40 This approach may still be used especially if the fistula is recurrent after previous attempts at fistulectomy. However, nowadays the preferred initial surgical treatment is by fistulectomy and primary closure with antibiotic coverage.41 Excision of all the major ducts is an alternative option depending on the circumstances (Table 17-5). When a localized periareolar abscess recurs at the previous site and a fistula is present, the preferred operation is fistulectomy, which has minimal complications and a high degree of success. However, when subareolar sepsis is diffuse rather than localized to one segment or when more than one fistula is present, total duct excision is the most expeditious approach. The first circumstance is seen in young women with squamous metaplasia of a single duct, whereas the latter circumstance is seen in older women with multiple ectatic ducts. Age is not always a reliable guide, however, and fistula excision is the preferred initial procedure for localized sepsis irrespective of age. Antibiotic therapy is useful for recurrent infection after fistula excision, and a 2- to 4-week course is recommended before total duct excision.
SUITABLE FOR FISTULECTOMY | SUITABLE FOR TOTAL DUCT EXCISION |
|---|---|
Small abscess localized to one segment | Large abscess affecting >50% of the areolar circumference |
Recurrence involving the same segment | Recurrence involving a different segment |
Mild or no nipple inversion | Marked nipple inversion |
Patient unconcerned about nipple inversion | Patient requests correction of nipple inversion |
Younger patient | Older patient |
No discharge from other ducts | Purulent discharge from other ducts |
No prior fistulectomy | Recurrence after fistulectomy |
More women request correction of congenital nipple inversion than request correction for the nipple inversion that occurs secondary to duct ectasia. Although the results are usually satisfactory, women seeking correction for cosmetic reasons should always be made aware of the surgical complications of altered nipple sensation, nipple necrosis, and postoperative fibrosis with nipple retraction. Because nipple inversion is a result of shortening of the subareolar ducts, a complete division of these ducts is necessary for permanent correction of the disorder.
RISK FACTORS FOR BREAST CANCER
Increased exposure to estrogen is associated with an increased risk for developing breast cancer, whereas reducing exposure is thought to be protective.42,43,44,45,46,47,48 Correspondingly, factors that increase the number of menstrual cycles, such as early menarche, nulliparity, and late menopause, are associated with increased risk. Moderate levels of exercise and a longer lactation period, factors that decrease the total number of menstrual cycles, are protective. The terminal differentiation of breast epithelium associated with a full-term pregnancy is also protective, so older age at first live birth is associated with an increased risk of breast cancer. Finally, there is an association between obesity and increased breast cancer risk. Because the major source of estrogen in postmenopausal women is the conversion of androstenedione to estrone by adipose tissue, obesity is associated with a long-term increase in estrogen exposure.
Nonhormonal risk factors include radiation exposure. Young women who receive mantle radiation therapy for Hodgkin’s lymphoma have a breast cancer risk that is 75 times greater than that of age-matched control subjects. Survivors of the atomic bomb blasts in Japan during World War II have a very high incidence of breast cancer, likely because of somatic mutations induced by the radiation exposure. In both circumstances, radiation exposure during adolescence, a period of active breast development, magnifies the deleterious effect. Studies also suggest that the risk of breast cancer increases as the amount of alcohol a woman consumes increases.49 Alcohol consumption is known to increase serum levels of estradiol. Finally, evidence suggests that long-term consumption of foods with a high fat content contributes to an increased risk of breast cancer by increasing serum estrogen levels.
The average lifetime risk of breast cancer for newborn U.S. females is 12%.50,51 The longer a woman lives without cancer, the lower her risk of developing breast cancer. Thus, a woman aged 50 years has an 11% lifetime risk of developing breast cancer, and a woman aged 70 years has a 7% lifetime risk of developing breast cancer. Because risk factors for breast cancer interact, evaluating the risk conferred by combinations of risk factors is difficult. There are several risk assessment models available to predict the risk of breast cancer. From the Breast Cancer Detection Demonstration Project, a mammography screening program conducted in the 1970s, Gail et al developed the model most frequently used in the United States, which incorporates age, age at menarche, age at first live birth, the number of breast biopsy specimens, any history of atypical hyperplasia, and number of first-degree relatives with breast cancer.52 It predicts the cumulative risk of breast cancer according to decade of life. To calculate breast cancer risk using the Gail model, a woman’s risk factors are translated into an overall risk score by multiplying her relative risks from several categories (Table 17-6). This risk score is then compared to an adjusted population risk of breast cancer to determine a woman’s individual or absolute risk. The output is a five-year risk and a lifetime risk of developing breast cancer. A software program incorporating the Gail model is available from the National Cancer Institute at . This model was recently modified to more accurately assess risk in African American women.52,53 The Gail model is the most widely used model in the United States. Gail and colleagues have also described a revised model that includes body weight and mammographic density but excludes age at menarche.54
VARIABLE | RELATIVE RISK |
|---|---|
Age at menarche (years) ≥14 12–13 <12 Number of biopsy specimens/history of benign breast disease, age <50 y 0 1 ≥2 Number of biopsy specimens/history of benign breast disease, age ≥50 y 0 1 ≥2 Age at first live birth (years) <20 y Number of first-degree relatives with history of breast cancer 0 1 ≥2 20–24 y Number of first-degree relatives with history of breast cancer 0 1 ≥2 25–29 y Number of first-degree relatives with history of breast cancer 0 1 ≥2 ≥30 y Number of first-degree relatives with history of breast cancer 0 1 ≥2 | 1.00 1.10 1.21 1.00 1.70 2.88 1.02 1.27 1.62 1.00 2.61 6.80 1.24 2.68 5.78 1.55 2.76 4.91 1.93 2.83 4.17 |
Claus et al, using data from the Cancer and Steroid Hormone Study, a case-control study of breast cancer, developed the other frequently used risk assessment model, which is based on assumptions about the prevalence of high-penetrance breast cancer susceptibility genes.55 Compared with the Gail model, the Claus model incorporates more information about family history but excludes other risk factors. The Claus model provides individual estimates of breast cancer risk according to decade of life based on presence of first- and second-degree relatives with breast cancer and their age at diagnosis. Risk factors that are less consistently associated with breast cancer (diet, use of oral contraceptives, lactation) or are rare in the general population (radiation exposure) are not included in either the Gail or Claus risk assessment model. Other models have been proposed that account for mammographic breast density in assessing breast cancer risk.54,56
Neither the Gail model nor the Claus model accounts for the risk associated with mutations in the breast cancer susceptibility genes BRCA1 and BRCA2 (described in detail below). The BRCAPRO model is a Mendelian model that calculates the probability that an individual is a carrier of a mutation in one of the breast cancer susceptibility genes based on their family history of breast and ovarian cancer.57 The probability that an individual will develop breast or ovarian cancer is derived from this mutation probability based on age-specific incidence curves for both mutation carriers and noncarriers.58 Use of the BRCAPRO model in the clinic is challenging since it requires input of all family history information regarding breast and ovarian cancer. The Tyrer-Cuzick model attempts to utilize both family history information and individual risk information. It uses the family history to calculate the probability that an individual carries a mutation in one of the breast cancer susceptibility genes and then the risk is adjusted based on personal risk factors, including age at menarche, parity, age at first live birth, age at menopause, history of atypical hyperplasia or LCIS, height and body mass index.59 Once a risk model has been utilized to assess breast cancer risk, this must be communicated to the individual and put into context with competing risk and medical comorbidities. This information can then be used to discuss options that are available to the individual for managing risk.
Several important medical decisions may be affected by a woman’s underlying risk of developing breast cancer.60,61,62,63,64,65,66,67,68 These decisions include when to use postmenopausal hormone replacement therapy, at what age to begin mammography screening or incorporate magnetic resonance imaging (MRI) screening, when to use tamoxifen to prevent breast cancer, and when to perform prophylactic mastectomy to prevent breast cancer. Postmenopausal hormone replacement therapy was widely prescribed in the 1980s and 1990s because of its effectiveness in controlling the symptoms of estrogen deficiency; namely, vasomotor symptoms such as hot flashes, night sweats and their associated sleep deprivation, osteoporosis, and cognitive changes. Furthermore, these hormone supplements were thought to reduce coronary artery disease as well. Use of combined estrogen and progesterone became standard for women who had not undergone hysterectomy, because unopposed estrogen increases the risk of uterine cancer. Concerns of prolonging a woman’s lifetime exposure to estrogen, coupled with conflicting data regarding the impact of these hormones on cardiovascular health, motivated the implementation of large-scale phase III clinical trials to definitively evaluate the risks vs. benefits of postmenopausal hormone replacement therapy. The Women’s Health Initiative was therefore designed by the National Institutes of Health as a series of clinical trials to study the effects of diet, nutritional supplements, and hormones on the risk of cancer, cardiovascular disease, and bone health in postmenopausal women. Findings from primary studies of postmenopausal hormone replacement therapy were released in 2002, demonstrating conclusively that breast cancer risk is threefold to fourfold higher after >4 years of use and there is no significant reduction in coronary artery or cerebrovascular risks. The Collaborative Group on Hormonal Factors in Breast Cancer combined and re-analyzed data from a number of studies totaling 52,705 women with breast cancer and 108,411 women without breast cancer. They found an increased risk of breast cancer with ever use of estrogen replacement therapy. They also reported increased risk among current users but not past users and risk increased with increasing duration of use of hormone replacement therapy.69 Cheblowski et al also reported from the WHI study that estrogen + progesterone increased the incidence of breast cancer.70 This was confirmed by the Million Women study which also showed that the increased risk was substantially greater for the combined estrogen + progesterone replacement therapy than other types of hormone replacement therapy.71
Routine use of screening mammography in women ≥50 years of age has been reported to reduce mortality from breast cancer by 25%.72 This reduction comes at an acceptable economic cost. More recently, there has been debate over the potential harms associated with breast screening.73 As a result the United Kingdom recently established an independent expert panel to review the published literature and estimate the benefits and harms associated with screening women >50 years in its national screening program.74 The expert panel estimated that an invitation to breast screening delivers about a 20% reduction in breast cancer mortality while at the same time the panel estimated that in women invited to screening, about 11% of the cancers diagnosed in their lifetime constitute over-diagnosis. Despite the overdiagnosis, the panel concluded that breast screening confers significant benefit and should continue. The use of screening mammography in women <50 years of age is more controversial for several reasons: (a) breast density is greater and screening mammography is less likely to detect early breast cancer (i.e., reduced sensitivity); (b) screening mammography results in more false-positive test findings (i.e., reduced specificity), which results in unnecessary biopsy specimens; and (c) younger women are less likely to have breast cancer (i.e., lower incidence), so fewer young women will benefit from screening.75,76 In the United States, on a population basis, however, the benefits of screening mammography in women between the ages of 40 and 49 years is still felt to outweigh the risks; although targeting mammography to women at higher risk of breast cancer improves the balance of risks and benefits and is the approach some health care systems have taken. In one study of women aged 40 to 49 years, an abnormal mammography finding was three times more likely to be cancer in a woman with a family history of breast cancer than in a woman without such a history. Furthermore, as noted previously in the section Risk Assessment Models, mounting data regarding mammographic breast density demonstrate an independent correlation with breast cancer risk. Incorporation of breast density measurements into breast cancer risk assessment models appears to be a promising strategy for increasing the accuracy of these tools. Unfortunately, widespread application of these modified models is hampered by inconsistencies in the reporting of mammographic density. Ultrasonography can also be used for breast cancer screening in women with dense breasts but there is no data available that the additional cancers detected with this modality reduce mortality from breast cancer.
Current recommendations by the United States Preventive Services Task Force are that women undergo biennial mammographic screening between the ages of 50 and 74 years.77 The American Cancer Society (ACS) continues to recommend annual mammography for women beginning at age 40 years to continue as long as she is in good health. In addition, a clinical breast examination by a health professional is recommended annually. The use of MRI for breast cancer screening is recommended by the ACS for women with a 20% to 25% or greater lifetime risk using risk assessment tools based mainly on family history, BRCA mutation carriers, those individuals who have a family member with a BRCA mutation who have not been tested themselves, individuals who received radiation to the chest between the ages of 10 to 30 years, and those individuals with a history of Li-Fraumeni syndrome, Cowden syndrome, or Bannayan-Riley-Ruvalcaba syndrome or those who have a first-degree relative with one of these syndromes. MRI is an extremely sensitive screening tool that is not limited by the density of the breast tissue as mammography is, however, its specificity is moderate leading to more false-positive events and the increased need for biopsy.
Tamoxifen, a selective estrogen receptor modulator, was the first drug shown to reduce the incidence of breast cancer in healthy women. There have been 4 prospective studies published evaluating tamoxifen vs. placebo for reducing the incidence of invasive breast cancer for women at increased risk. The largest trial was the Breast Cancer Prevention Trial (NSABP P-01) which randomly assigned >13,000 women with a 5-year Gail relative risk of breast cancer of 1.66% or higher or LCIS to receive tamoxifen or placebo. After a mean follow-up period of 4 years, the incidence of breast cancer was reduced by 49% in the group receiving tamoxifen.60 The decrease was evident only in ER-positive breast cancers with no significant change in ER-negative tumors. The Royal Marsden Hospital Tamoxifen Chemoprevention Trial78, the Italian Tamoxifen Prevention Trial79, and the International Breast Cancer Intervention Study I (IBIS-I) trial all80 showed a reduction in ER-positive breast cancers with the use of tamoxifen compared with placebo. There was no effect on mortality; however, the trials were not powered to assess either breast cancer mortality or all-cause mortality events. The adverse events were similar in all 4 randomized trials including an increased risk of endometrial cancer, thromboembolic events, cataract formation, and vasomotor disturbances in individuals receiving tamoxifen.
Tamoxifen therapy currently is recommended only for women who have a Gail relative risk of 1.66% or higher, who are aged 35 to 59, women over the age of 60 or women with a diagnosis of LCIS or atypical ductal or lobular hyperplasia. In addition, deep vein thrombosis occurs 1.6 times as often, pulmonary emboli 3.0 times as often, and endometrial cancer 2.5 times as often in women taking tamoxifen. The increased risk for endometrial cancer is restricted to early stage cancers in postmenopausal women. Cataract surgery is required almost twice as often among women taking tamoxifen. Gail et al subsequently developed a model that accounts for underlying risk of breast cancer as well as comorbidities to determine the net risk-benefit ratio of tamoxifen use for chemoprevention.81
The NSABP completed a second chemoprevention trial, designed to compare tamoxifen and raloxifene for breast cancer risk reduction in high-risk postmenopausal women. Raloxifene, another selective estrogen receptor modulator, was selected for the experimental arm in this follow-up prevention trial because its use in managing postmenopausal osteoporosis suggested that it might be even more effective at breast cancer risk reduction, but without the adverse effects of tamoxifen on the uterus. The P-2 trial, the Study of Tamoxifen and Raloxifene (known as the STAR trial), randomly assigned 19,747 postmenopausal women at high-risk for breast cancer to receive either tamoxifen or raloxifene. The initial report of the P-2 trial showed the two agents were nearly identical in their ability to reduce breast cancer risk, but raloxifene was associated with a more favorable adverse event profile.82 An updated analysis revealed that raloxifene maintained 76% of the efficacy of tamoxifen in prevention of invasive breast cancer with a more favorable side effect profile. The risk of developing endometrial cancer was significantly higher with tamoxifen use at longer follow-up.83 Although tamoxifen has been shown to reduce the incidence of LCIS and DCIS, raloxifene did not have an effect on the frequency of these diagnoses.
Aromatase inhibitors (AIs) have been shown to be more effective than tamoxifen in reducing the incidence of contralateral breast cancers in postmenopausal women receiving AIs for adjuvant treatment of invasive breast cancer. The MAP.3 trial was the first study to evaluate an AI as a chemopreventive agent in postmenopausal women at high risk for breast cancer. The trial randomized 4,560 women to exemestane 25 mg daily vs. placebo for five years. After a median follow-up of 35 months, exemestane was shown to reduce invasive breast cancer incidence by 65%. Side effect profiles demonstrated more grade 2 or higher arthritis and hot flashes in patients taking exemestane.84 The IBIS II trial has recruited 6,000 patients randomized to the non-steroidal aromatase inhibitor, anastrozole, vs. placebo with a further randomization to bisphosphonate or not based on bone density.85 The trial also had an initial sub-study which looked at the effect of the aromatase inhibitor on cognitive function and reported no adverse effects.86 The American Society of Clinical Oncology recently updated recommendations for chemoprevention in women at increased risk of breast cancer as did the U.S. Preventive Services Task Force. Both groups recommend offering tamoxifen to women at increased risk for breast cancer or raloxifene to postmenopausal women who are noted to be at increased risk.87,88 The discussion with an individual patient should include risk assessment and potential risks and benefits with each agent.
A retrospective study of women at high risk for breast cancer found that prophylactic mastectomy reduced their risk by >90%.62 However, the effects of prophylactic mastectomy on the long-term quality of life are poorly quantified. A study involving women who were carriers of a breast cancer susceptibility gene (BRCA) mutation found that the benefit of prophylactic mastectomy differed substantially according to the breast cancer risk conferred by the mutations. For women with an estimated lifetime risk of 40%, prophylactic mastectomy added almost 3 years of life, whereas for women with an estimated lifetime risk of 85%, prophylactic mastectomy added >5 years of life.66 Domchek et al evaluated a cohort of BRCA1/2 mutation carriers who were followed prospectively and reported on outcomes with risk-reducing surgery.89 They found that risk-reducing mastectomy was highly effective at preventing breast cancer in both BRCA1 and 2 mutation carriers. Risk-reducing salpingo-oophorectomy was highly effective at reducing the incidence of ovarian cancer and breast cancer in BRCA mutation carriers and was associated with a reduction in breast cancer-specific mortality, ovarian cancer-specific mortality, and all-cause mortality. While studies of bilateral prophylactic or risk-reducing mastectomy have reported dramatic reductions in breast cancer incidence among those without known BRCA mutations, there is little data to support a survival benefit. Another consideration is that while most patients are satisfied with their decision to pursue risk-reducing surgery, some are dissatisfied with the cosmetic outcomes mostly due to reconstructive issues.
Up to 5% of breast cancers are caused by inheritance of germline mutations such as BRCA1 and BRCA2, which are inherited in an autosomal dominant fashion with varying degrees of penetrance (Table 17-7).90,91,92,93,94,95,96BRCA1 is located on chromosome arm 17q, spans a genomic region of approximately 100 kilobases (kb) of DNA, and contains 22 coding exons for 1863 amino acids. Both BRCA1 and BRCA2 function as tumor-suppressor genes, and for each gene, loss of both alleles is required for the initiation of cancer. Data accumulated since the isolation of the BRCA1 gene suggest a role in transcription, cell-cycle control, and DNA damage repair pathways. More than 500 sequence variations in BRCA1 have been identified. It now is known that germline mutations in BRCA1 represent a predisposing genetic factor in as many as 45% of hereditary breast cancers and in at least 80% of hereditary ovarian cancers. Female mutation carriers have been reported to have up to a 85% lifetime risk (for some families) for developing breast cancer and up to a 40% lifetime risk for developing ovarian cancer. The initial families reported had high penetrance and subsequently the average lifetime risk has been reported to lie between 60%–70%. Breast cancer susceptibility in these families appears as an autosomal dominant trait with high penetrance. Approximately 50% of children of carriers inherit the trait. In general, BRCA1-associated breast cancers are invasive ductal carcinomas, are poorly differentiated, are in the majority hormone receptor negative and have a triple receptor negative (immunohistochemical profile: ER-negative, PR-negative and HER-2-negative) or basal phenotype (based on gene expression profiling). BRCA1-associated breast cancers have a number of distinguishing clinical features, such as an early age of onset compared with sporadic cases; a higher prevalence of bilateral breast cancer; and the presence of associated cancers in some affected individuals, specifically ovarian cancer and possibly colon and prostate cancers.
Sporadic breast cancer | 65%–75% |
Familial breast cancer | 20%–30% |
Hereditary breast cancer | 5%–10% |
BRCA1a | 45% |
BRCA2 | 35% |
p53a (Li-Fraumeni syndrome) | 1% |
STK11/LKB1a (Peutz-Jeghers syndrome) | <1% |
PTENa (Cowden disease) | <1% |
MSH2/MLH1a (Muir-Torre syndrome) | <1% |
ATMa (Ataxia-telangiectasia) | <1% |
Unknown | 20% |
Several founder mutations have been identified in BRCA1. The two most common mutations are 185delAG and 5382insC, which account for 10% of all the mutations seen in BRCA1. These two mutations occur at a 10-fold higher frequency in the Ashkenazi Jewish population than in non-Jewish caucasians. The carrier frequency of the 185delAG mutation in the Ashkenazi Jewish population is 1% and, along with the 5382insC mutation, accounts for almost all BRCA1 mutations in this population. Analysis of germline mutations in Jewish and non-Jewish women with early-onset breast cancer indicates that 20% of Jewish women who develop breast cancer before age 40 years carry the 185delAG mutation. There are founder BRCA1 mutations in other populations including, among others, Dutch, Polish, Finnish, and Russian populations.97,98,99,100,101
BRCA2 is located on chromosome arm 13q and spans a genomic region of approximately 70 kb of DNA. The 11.2-kb coding region contains 26 coding exons.90,91,92,93,94,95,96 It encodes a protein of 3418 amino acids. The BRCA2 gene bears no homology to any previously described gene, and the protein contains no previously defined functional domains. The biologic function of BRCA2 is not well defined, but like BRCA1, it is postulated to play a role in DNA damage response pathways. BRCA2 messenger RNA also is expressed at high levels in the late G1 and S phases of the cell cycle. The kinetics of BRCA2 protein regulation in the cell cycle is similar to that of BRCA1 protein, which suggests that these genes are coregulated. The mutational spectrum of BRCA2 is not as well established as that of BRCA1. To date, >250 mutations have been found. The breast cancer risk for BRCA2 mutation carriers is close to 85%, and the lifetime ovarian cancer risk, while lower than for BRCA1, is still estimated to be close to 20%. Breast cancer susceptibility in BRCA2 families is an autosomal dominant trait and has a high penetrance. Approximately 50% of children of carriers inherit the trait. Unlike male carriers of BRCA1 mutations, men with germline mutations in BRCA2 have an estimated breast cancer risk of 6%, which represents a 100-fold increase over the risk in the general male population. BRCA2-associated breast cancers are invasive ductal carcinomas, which are more likely to be well differentiated and to express hormone receptors than are BRCA1-associated breast cancers. BRCA2-associated breast cancer has a number of distinguishing clinical features, such as an early age of onset compared with sporadic cases, a higher prevalence of bilateral breast cancer, and the presence of associated cancers in some affected individuals, specifically ovarian, colon, prostate, pancreatic, gallbladder, bile duct, and stomach cancers, as well as melanoma. A number of founder mutations have been identified in BRCA2. The 6174delT mutation is found in Ashkenazi Jews with a prevalence of 1.2% and accounts for 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women.102 Another BRCA2 founder mutation, 999del5, is observed in Icelandic and Finnish populations while more recently 3036delACAA has been observed in a number of Spanish families.103,104,105
Identifying hereditary risk for breast cancer is a four-step process that includes: (a) obtaining a complete, multigenerational family history, (b) assessing the appropriateness of genetic testing for a particular patient, (c) counseling the patient, and (d) interpreting the results of testing.106 Genetic testing should not be offered in isolation, but only in conjunction with patient education and counseling, including referral to a genetic counselor. Initial determinations include whether the individual is an appropriate candidate for genetic testing and whether genetic testing will be informative for personal and clinical decision making. A thorough and accurate family history is essential to this process, and the maternal and paternal sides of the family are both assessed, because 50% of the women with a BRCA mutation have inherited the mutation from their fathers. To help clinicians advise women about genetic testing, statistically based models that determine the probability that an individual carries a BRCA mutation have been developed. A method for calculating carrier probability which has been demonstrated to have acceptable performance (i.e., both in terms of calibration and discrimination) such as the Manchester scoring system and BODICEA should be used to offer referral to a specialist genetic clinic. A hereditary risk of breast cancer is considered if a family includes Ashkenazi Jewish heritage; a first-degree relative with breast cancer before age 50; a history of ovarian cancer at any age in the patient or first- or second-degree relative with ovarian cancer; breast and ovarian cancer in the same individual; two or more first- or second-degree relatives with breast cancer at any age; patient or relative with bilateral breast cancer; and male breast cancer in a relative at any age.107 The threshold for genetic testing is lower in individuals who are members of ethnic groups in whom the mutation prevalence is increased.
Appropriate counseling for the individual being tested for a BRCA mutation is strongly recommended, and documentation of informed consent is required.106,108 The test that is clinically available for analyzing BRCA mutations is gene sequence analysis. In a family with a history suggestive of hereditary breast cancer and no previously tested member, the most informative strategy is first to test an affected family member. This person undergoes complete sequence analysis of both the BRCA1 and BRCA2 genes. If a mutation is identified, relatives are usually tested only for that specific mutation. An individual of Ashkenazi Jewish ancestry is tested initially for the three specific mutations that account for hereditary breast and ovarian cancer in that population. If results of that test are negative, it may then be appropriate to fully analyze the BRCA1 and BRCA2 genes.
A positive test result is one that discloses the presence of a BRCA mutation that interferes with translation or function of the BRCA protein. A woman who carries a deleterious mutation has a breast cancer risk of up to 85% (in some families) as well as a greatly increased risk of ovarian cancer. A negative test result is interpreted according to the individual’s personal and family history, especially whether a mutation has been previously identified in the family, in which case the woman is generally tested only for that specific mutation. If the mutation is not present, the woman’s risk of breast or ovarian cancer may be no greater than that of the general population. In addition, no BRCA mutation can be passed on to the woman’s children. In the absence of a previously identified mutation, a negative test result in an affected individual generally indicates that a BRCA mutation is not responsible for the familial cancer. However, the possibility remains of an unusual abnormality in one of these genes that cannot yet be identified through clinical testing. It also is possible that the familial cancer is indeed caused by an identifiable BRCA mutation but that the individual tested had sporadic cancer, a situation known as phenocopy. This is especially possible if the individual tested developed breast cancer close to the age of onset of the general population (age 60 years or older) rather than before age 50 years, as is characteristic of BRCA mutation carriers. Overall, the false-negative rate for BRCA mutation testing is <5%. Some test results, especially when a single base-pair change (missense mutation) is identified, may be difficult to interpret. This is because single base-pair changes do not always result in a nonfunctional protein. Thus, missense mutations not located within critical functional domains, or those that cause only minimal changes in protein structure, may not be disease associated and are usually reported as indeterminate results. In communicating indeterminate results to women, care must be taken to relay the uncertain cancer risk associated with this type of mutation and to emphasize that ongoing research might clarify its meaning. In addition, testing other family members with breast cancer to determine if a genetic variant tracks with their breast cancer may provide clarification as to its significance. Indeterminate genetic variance currently accounts for 12% of the test results.
Concern has been expressed that the identification of hereditary risk for breast cancer may interfere with access to affordable health insurance. This concern refers to discrimination directed against an individual or family based solely on an apparent or perceived genetic variation from the normal human genotype. The Health Insurance Portability and Accountability Act of 1996 (HIPAA) made it illegal in the United States for group health plans to consider genetic information as a preexisting condition or to use it to deny or limit coverage. Most states also have passed laws that prevent genetic discrimination in the provision of health insurance. In addition, individuals applying for health insurance are not required to report whether relatives have undergone genetic testing for cancer risk, only whether those relatives have actually been diagnosed with cancer. Currently there is little documented evidence of genetic discrimination resulting from findings of available genetic tests.
Risk management strategies for BRCA1 and BRCA2 mutation carriers include the following:
Risk-reducing mastectomy and reconstruction
Risk-reducing salpingo-oophorectomy
Intensive surveillance for breast and ovarian cancer
Chemoprevention
Although removal of breast tissue reduces the likelihood that BRCA1 and BRCA2 mutation carriers will develop breast cancer, mastectomy does not remove all breast tissue and women continue to be at risk because a germline mutation is present in any remaining breast tissue. For postmenopausal BRCA1 and BRCA2 mutation carriers who have not had a mastectomy, it may be advisable to avoid hormone replacement therapy, because no data exist regarding the effect of the therapy on the penetrance of breast cancer susceptibility genes. Because breast cancers in BRCA mutation carriers have the same mammographic appearance as breast cancers in noncarriers, a screening mammogram is likely to be effective in BRCA mutation carriers, provided it is performed and interpreted by an experienced radiologist with a high level of suspicion. Present screening recommendations for BRCA mutation carriers who do not undergo risk-reducing mastectomy include clinical breast examination every 6 months and mammography every 12 months beginning at age 25 years, because the risk of breast cancer in BRCA mutation carriers increases after age 30 years. Recent attention has been focused on the use MRI for breast cancer screening in high-risk individuals and known BRCA mutation carriers. MRI appears to be more sensitive at detecting breast cancer in younger women with dense breasts.109 However, as noted previously, MRI does lead to the detection of benign breast lesions that cannot easily be distinguished from malignancy, and these false-positive events can result in more interventions, including biopsy specimens. The current recommendations from the American Cancer Society are for annual MRI in women with a 20% to 25% or greater lifetime risk of developing breast cancer, including women with a strong family history of breast or ovarian cancer and women who were treated for Hodgkin’s disease in their teens or early twenties.110 Despite a 49% reduction in the overall incidence of breast cancer and a 69% reduction in the incidence of estrogen receptor positive tumors in high-risk women taking tamoxifen reported in the NSABP P1 trial, there is insufficient evidence to recommend the use of tamoxifen uniformly for BRCA1 mutation carriers.60 Cancers arising in BRCA1 mutation carriers are usually high grade and are most often hormone receptor negative. Approximately 66% of BRCA1-associated DCIS lesions are estrogen receptor negative, which suggests early acquisition of the hormone-independent phenotype. In the NSABP P1 trial there was a 62% reduction in the incidence of breast cancer in BRCA2 carriers, similar to the overall reduction seen in the P1 trial. In contrast, there was no reduction seen in breast cancer incidence in BRCA1 carriers who started tamoxifen in P1 age 35 years or older.111 Tamoxifen appears to be more effective at preventing estrogen receptor-positive breast cancers.
The risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers ranges from 20% to 40%, which is 10 times higher than that in the general population. Risk-reducing salpingo-oophorectomy is a reasonable prevention option in mutation carriers. In women with a documented BRCA1 or BRCA2 mutation, consideration for bilateral risk-reducing salpingo-ophorectomy should be between the ages of 35 and 40 years at the completion of childbearing. Removing the ovaries reduces the risk of ovarian cancer and breast cancer when performed in premenopausal BRCA mutation carriers. Hormone replacement therapy is discussed with the patient at the time of oophorectomy. The Cancer Genetics Studies Consortium recommends yearly transvaginal ultrasound timed to avoid ovulation and annual measurement of serum cancer antigen 125 levels beginning at age 25 years as the best screening modalities for ovarian carcinoma in BRCA mutation carriers who have opted to defer prophylactic surgery.
Other hereditary syndromes associated with an increased risk of breast cancer include Cowden disease (PTEN mutations, in which cancers of the thyroid, GI tract, and benign skin and subcutaneous nodules are also seen), Li-Fraumeni syndrome (p53 mutations, also associated with sarcomas, lymphomas, and adrenocortical tumors), and syndromes of breast and melanoma.
EPIDEMIOLOGY AND NATURAL HISTORY OF BREAST CANCER
Breast cancer is the most common site-specific cancer in women and is the leading cause of death from cancer for women aged 20 to 59 years. It accounts for 29% of all newly diagnosed cancers in females and is responsible for 14% of the cancer-related deaths in women. It is predicted that approximately 234,580 breast cancers will be diagnosed in the United States in 2013 and that 40,030 individuals will die from breast cancer.112 Breast cancer was the leading cause of cancer-related mortality in women until 1987, when it was surpassed by lung cancer. In the 1970s, the probability that a woman in the United States would develop breast cancer at some point in her lifetime was estimated at 1 in 13; in 1980 it was 1 in 11; and in 2004 it was 1 in 8. Cancer registries in Connecticut and upper New York State document that the age-adjusted incidence of new breast cancer cases had steadily increased since the mid-1940s. The incidence in the United States, based on data from nine Surveillance, Epidemiology, and End Results (SEER) registries, has been decreasing by 23% per year since 2000. The increase had been approximately 1% per year from 1973 to 1980, and there was an additional increase in incidence of 4% between 1980 and 1987, which was characterized by frequent detection of small primary cancers. The increase in breast cancer incidence occurred primarily in women ≥55 years and paralleled a marked increase in the percentage of older women who had mammograms taken. At the same time, incidence rates for regional metastatic disease dropped and breast cancer mortality declined. From 1960 to 1963, 5-year overall survival rates for breast cancer were 63% and 46% in white and African American women, respectively, whereas the rates for 1981 to 1983 were 78% and 64%, respectively. For 2002 to 2008 rates were 92% and 78%, respectively.
There is a 10-fold variation in breast cancer incidence among different countries worldwide. Cyprus and Malta have the highest age-adjusted mortality for breast cancer (29.6 per 100,000 population), whereas Haiti has the lowest (2.0 deaths per 100,000 population). The United States has an age-adjusted mortality for breast cancer of 19.0 cases per 100,000 population. Women living in less-industrialized nations tend to have a lower incidence of breast cancer than women living in industrialized countries, although Japan is an exception. In the United States, Mormons, Seventh Day Adventists, American Indians, Alaska natives, Hispanic/Latina Americans, and Japanese and Filipino women living in Hawaii have a below-average incidence of breast cancer, whereas nuns (due to nulliparity) and Ashkenazi Jewish women have an above-average incidence.
The incidence rates of breast cancer increased in most countries through the 1990s. Since the estimates for 1990, there was an overall increase in incidence rates of approximately 0.5% annually. It was predicted that there would be approximately 1.4 million new cases in 2010. The cancer registries in China have noted annual increases in incidence of up to 3% to 4%, and in eastern Asia, increases are similar.
Recent data from the SEER program reveal declines in breast cancer incidence over the past decade, and this is widely attributed to decreased use of hormone replacement therapy as a consequence of the Women’s Health Initiative reports.113
Breast cancer burden has well-defined variations by geography, regional lifestyle, and racial or ethnic background.114 In general, both breast cancer incidence and mortality are relatively lower among the female populations of Asia and Africa, relatively underdeveloped nations, and nations that have not adopted the Westernized reproductive and dietary patterns. In contrast, European and North American women and women from heavily industrialized or westernized countries have a substantially higher breast cancer burden. These international patterns are mirrored in breast cancer incidence and mortality rates observed for the racially, ethnically, and culturally diverse population of the United States.115
Although often related, the factors that influence breast cancer incidence may differ from those that affect mortality. Incidence rates are lower among populations that are heavily weighted with women who begin childbearing at young ages and who have multiple full-term pregnancies followed by prolonged lactation. These are features that characterize many underdeveloped nations and also many eastern nations. Breast cancer mortality rates should be lower in populations that have a lower incidence, but the mortality burden will simultaneously be adversely affected by the absence of effective mammographic screening programs for early detection and diminished access to multidisciplinary cancer treatment programs. These features are likely to account for much of the disproportionate mortality risks that are seen in underdeveloped nations. Similar factors probably account for differences in breast cancer burden observed among the various racial and ethnic groups within the United States. Interestingly, breast cancer incidence and mortality rates rise among second- and third-generation Asian Americans as they adopt Western lifestyles.
Disparities in breast cancer survival among subsets of the American population are generating increased publicity because they are closely linked to disparities in socioeconomic status. Poverty rates and proportions of the population that lack health care insurance are two to three times higher among minority racial and ethnic groups such as African Americans and Hispanic/Latino Americans. These socioeconomic disadvantages create barriers to effective breast cancer screening and result in delayed breast cancer diagnosis, advanced stage distribution, inadequacies in comprehensive treatment, and ultimately increased mortality rates. Furthermore, the rapid growth in the Hispanic population is accompanied by increasing problems in health education because of linguistic barriers between physicians and recently immigrated, non-English-speaking patients. Recent studies also are documenting inequities in the treatments delivered to minority breast cancer patients, such as increased rates of failure to provide systemic therapy, use of sentinel lymph node dissection, and breast reconstruction. Some of the treatment delivery disparities are related to inadequately controlled comorbidities (such as hypertension and diabetes), which are more prevalent in minority populations. However, some studies that adjust for these factors report persistent and unexplained unevenness in treatment recommendations. It is clear that breast cancer disparities associated with racial or ethnic background have a multifactorial cause, and improvements in outcome will require correction of many public health problems at both the patient and provider levels.
Advances in the ability to characterize breast cancer subtypes and the genetics of the disease are now provoking speculation regarding possible hereditary influences on breast cancer risk that are related to racial or ethnic ancestry.116 These questions become particularly compelling when one looks at disparities in breast cancer burden between African Americans and Caucasians. Lifetime risk of breast cancer is lower for African Americans, yet a paradoxically increased breast cancer mortality risk also is seen. African Americans also have a younger age distribution for breast cancer; among women <45 years of age, breast cancer incidence is highest among African Americans compared to other subsets of the American population. Lastly and most provocatively, African American women of all ages have notably higher incidence rates for estrogen receptor-negative tumors. These same patterns of disease are seen in contemporary female populations of western, sub-Saharan Africa, who are likely to share ancestry with African American women as a consequence of the Colonial-era slave trade. Interestingly, male breast cancer also is seen with increased frequency among both African Americans and Africans.
Bloom and colleagues described the natural history of breast cancer based on the records of 250 women with untreated breast cancers who were cared for on charity wards in the Middlesex Hospital, London, between 1805 and 1933. The median survival of this population was 2.7 years after initial diagnosis (Fig. 17-13).117 The 5- and 10-year survival rates for these women were 18.0% and 3.6%, respectively. Only 0.8% survived for 15 years or longer. Autopsy data confirmed that 95% of these women died of breast cancer, whereas the remaining 5% died of other causes. Almost 75% of the women developed ulceration of the breast during the course of the disease. The longest surviving patient died in the nineteenth year after diagnosis.
Figure 17-13.
Survival of women with untreated breast cancer compared with natural survival. (Reproduced with permission from Bloom HJG, Richardson WW, Harries EJ. Natural history of untreated breast cancer [1803–1933]: Comparison of untreated and treated cases according to histological grade of malignancy. Br Med J. With permission from BMJ Publishing Group, Ltd.)
More than 80% of breast cancers show productive fibrosis that involves the epithelial and stromal tissues. With growth of the cancer and invasion of the surrounding breast tissues, the accompanying desmoplastic response entraps and shortens Cooper’s suspensory ligaments to produce a characteristic skin retraction. Localized edema (peaud’orange) develops when drainage of lymph fluid from the skin is disrupted. With continued growth, cancer cells invade the skin, and eventually ulceration occurs. As new areas of skin are invaded, small satellite nodules appear near the primary ulceration. The size of the primary breast cancer correlates with disease-free and overall survival, but there is a close association between cancer size and axillary lymph node involvement (Fig. 17-14). In general, up to 20% of breast cancer recurrences are local-regional, >60% are distant, and 20% are both local-regional and distant.
Figure 17-14.
A. Overall survival for women with breast cancer according to axillary lymph node status. The time periods are years after radical mastectomy. (Reproduced with permission from Valagussa P, et al: Patterns of relapse and survival following radical mastectomy. Analysis of 716 consecutive patients. Cancer. 1978;11:1170. Copyright © American Cancer Society. This material is reproduced with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.) B. Risk of metastases according to breast cancer volume and diameter. (Reproduced with permission from Koscielny S et al. Breast cancer: Relationship between the size of the primary tumour and the probability of metastatic dissemination. Br J Cancer. 1984;49:709.)
As the size of the primary breast cancer increases, some cancer cells are shed into cellular spaces and transported via the lymphatic network of the breast to the regional lymph nodes, especially the axillary lymph nodes. Lymph nodes that contain metastatic cancer are at first ill-defined and soft but become firm or hard with continued growth of the metastatic cancer. Eventually the lymph nodes adhere to each other and form a conglomerate mass. Cancer cells may grow through the lymph node capsule and fix to contiguous structures in the axilla, including the chest wall. Typically, axillary lymph nodes are involved sequentially from the low (level I) to the central (level II) to the apical (level III) lymph node groups. Approximately 95% of the women who die of breast cancer have distant metastases, and traditionally the most important prognostic correlate of disease-free and overall survival was axillary lymph node status (see Fig. 17-14A). Women with node-negative disease had less than a 30% risk of recurrence, compared with as much as a 75% risk for women with node-positive disease.
At approximately the twentieth cell doubling, breast cancers acquire their own blood supply (neovascularization). Thereafter, cancer cells may be shed directly into the systemic venous blood to seed the pulmonary circulation via the axillary and intercostal veins or the vertebral column via Batson’s plexus of veins, which courses the length of the vertebral column. These cells are scavenged by natural killer lymphocytes and macrophages. Successful implantation of metastatic foci from breast cancer predictably occurs after the primary cancer exceeds 0.5 cm in diameter, which corresponds to the twenty-seventh cell doubling. For 10 years after initial treatment, distant metastases are the most common cause of death in breast cancer patients. For this reason, conclusive results cannot be derived from breast cancer trials until at least 5 to 10 years have elapsed. Although 60% of the women who develop distant metastases will do so within 60 months of treatment, metastases may become evident as late as 20 to 30 years after treatment of the primary cancer.118 Patients with estrogen receptor negative breast cancers are proportionately more likely to develop recurrence in the first 3 to 5 years, whereas those with estrogen receptor positive tumors have a risk of developing recurrence which drops off more slowly beyond 5 years than is seen with ER negative tumors.119
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree