The Barriers of the Upper Gastrointestinal Tract
Introduction
|
The common acid related diseases of the upper gastrointestinal tract can be considered as primarily due to a defect in barrier function of the esophageal epithelium or primarily the lower esophageal sphincter in the case of GERD or of the gastric mucosal or duodenal epithelium in the case of gastric or duodenal ulcers. The defect may be natural or induced, for example, by infection with H. pylori. In rarer situations, such as Zollinger-Ellison syndrome, the extraordinary levels of acid may overwhelm otherwise normal barriers. The lower esophageal sphincter will be discussed in some detail in the section on GERD, and this introduction to acid related diseases will focus on the acidresistance properties of the esophageal, gastric, and duodenal epithelia.
Historical aspects
Once it had become apparent that digestion was an active process involving acid and pepsin that was initiated in the stomach, a further fundamental question arose. Why did the stomach not digest itself? This problem had long been pondered, and Archibald Pitcairn, the great Iatromathematician, had himself questioned the Iatrochemists rigorously on this issue: “…Why on the digestion of food on the stomach, which is as easily digestible as the food, yet the stomach itself should not be dissolved?” No reasonable answer was forthcoming, and the pundits, such as John Hunter, could only perseverate on the existence of a putative vital force that maintained the gastric wall intact under the circumstances of digestion. Issues such as the existence of a “locus minoris resistentia”
were entertained to explain ulceration and embrace the concept that this was indeed local digestion of the stomach mucosa.
were entertained to explain ulceration and embrace the concept that this was indeed local digestion of the stomach mucosa.
Nevertheless, it seemed difficult to understand why food in the lumen of the stomach would be broken up whereas the wall of the stomach remained intact. The invocation of “vital spirit” was of little satisfaction and represented a regression to archaic views on the nature of digestion. Further reflection on the matter resulted in the recognition that the stomach wall itself must harbor an intrinsic “force” capable of resisting digestion. Indeed, the attractive concept that a breakdown of such an intrinsic mechanism might be responsible for the development of ulcers or even neoplasia became a source of considerable speculation. Nevertheless, little evidence existed to delineate the specific nature of the mechanism responsible for the generation of mucosal protection from acid, pepsin, and even noxious agents that might be ingested.
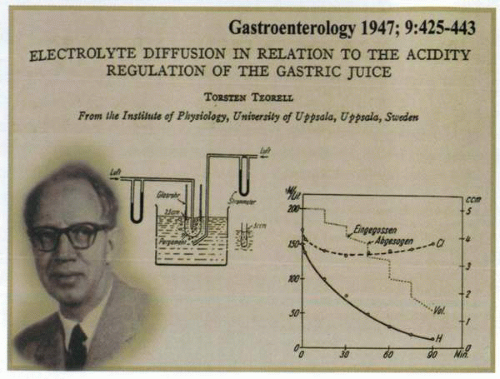
In the early 1930s, Torsten Teorell had begun to examine the permeability characteristics of gastric mucosa by suggesting that H+ diffused back through the mucosa in exchange for Na+. Thus, issues had arisen as to whether acid was neutralized within the lumen or after diffusion into the mucosa in the interstitial fluid. An alternative hypothesis had been that the bicarbonate content of gastric secretion might be responsible for some of the acid that was neutralized. Nevertheless, the ability of the stomach to retain the acid it secreted under normal circumstances without digesting itself once again raised the concept of the existence of a “gastric mucosal barrier.” Teorell began the delineation of the barrier properties by demonstrating that unionized organic acids, but not ionized mineral acids, would disappear rapidly from the gastric contents by diffusing into the mucosa. Although not fully resolving the issue, his concept of a diffusion process in the mucosa had by 1947 initiated the formal evaluation of the intrinsic mechanisms available to deal with back diffusion of acid into the interstitium of the stomach.
In 1955, Davenport and Code published a series of experiments under the title The Functional Significance of Gastric Mucosal Barrier to Sodium and thus initiated the first formal usage of the term barrier. They described the results of the relative rates of disappearance of radiolabeled 42K+, 22Na+, thiocyanate, or 3H2O from solutions in contact with oxyntic mucosa. They observed that when the gastric contents were neutral, 3H2O disappeared rapidly but 42K+ slowly and 22Na+ at an intermediate rate. However, when the gastric contents were acid, or gastric secretion was stimulated by histamine, the rates of disappearance of THO and 42K+ were unaffected, whereas the rates of disappearance of 22Na+ fell to zero. In recognition of the significance of this observation, Code commented presciently on this subject in the manuscript entitled The Barrier Offered by the Gastric Mucosa to Sodium.
Further experiments in this area were designed to identify the site of this zone and resulted in proposals that it was mucus that construed the barrier function. Proof for this, however, was difficult to obtain, and a reconfiguration of this proposal by others resulted in the hypothesis that bicarbonate secreted by the gastric mucosa neutralized acid and thus created a biochemical barrier. Further elaboration of this line of work generated much discussion about the “unstirred layer” and held that the modest concentration of bicarbonate held in this would produce a barrier of both functional and physical significance to acid. In a fashion reminiscent of the determination of the physical aura produced by plants, the size of this barrier was then measured and its certain presence fixed in the minds of the believers. Davenport in later work further explored the concept of the gastric mucosal barrier by seeking to damage it with various agents including eugenol, fatty acids, and alcohol, and was able to prove that back diffusion into the mucosa of acid occurred under such conditions. These observations were substantiated by anatomic evidence of increased mucosal permeability by demonstrating damage to the surface epithelial cells and underlying tissues, including vessels. Some support was thus provided for the important association of barrier breaking and its relationship to acute mucosal ulceration and stress bleeding.
An immense body of work was then undertaken by diverse investigators to identify the effects of hypoxia, acid, bile salts, aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), prostaglandins, etc., on the integrity of the barrier. The commutations and permutations of these studies achieved little except to further document the existence of the mucosal barrier and the ability of a variety of agents to break it. Unfortunately, this did little to identify the precise site and mechanism of the phenomenon. The summation of much work established that the barrier represented a generalized function common to a polarized epithelial cell system and that acids in an unionized state and fat-solubilizing agents were capable of acting as barrier-breaking agents. Breaking the barrier in itself was not sufficient but represented an initial step in the process of mucosal injury. A subsequent cascade of physiologic events involving back diffusion of acid with liberation of histamine and the activation of pepsinogen was involved.
The exploration of the multiplicity of associated phenomena provided further complex information regarding the fact that the barrier might not be a single entity but represent a multiplicity of physiologic protective mechanisms. The more recent use of sophisticated techniques to investigate cell function has determined the relevance of tight junctions and identified the specific property of the apical membrane of the gastric mucosal cells as being of vital importance in maintaining barrier function.
Davenport and Code thus deserve credit for extending the word barrier to include the property of the mucosa governing its permeability to H+. In subsequent years, the word would become a term broadly used to designate permeability of the mucosa in general. Fromm, in an evaluation of the entire subject, was to later remark that barrier and barrier breakers were simply catch phrases that, “while novel and clever, reflect our ignorance.” Davenport himself, in summarizing his accomplishments in the area, published them in a book whimsically entitled The Gastric Mucosal Barrier: A Swan Song.
The esophageal epithelium
Malfunction of the lower esophageal sphincter results in acid reflux into the esophagus. The acid load presented to the esophagus is likely to be the major determinant of heartburn or erosion. Reflux is mainly episodic, so that the esophagus is not often presented with a continuous acid load. Expulsion of the refluxate back into the stomach shortens the time of exposure.
The volume of reflux is also an important parameter for estimation of the acid load on the esophageal epithelium. Finally, the pH of the gastric contents that are refluxed into the esophagus also determines the possible damage. Intraesophageal pH monitoring provides an approximate measure of the acid load to the lower esophagus in terms of the pH of refluxate and dwell time of the acid solution and is probably the most useful semiquantitative measure of the degree of exposure of the esophageal epithelium.
The human esophageal epithelium is a multilayered, stratified squamous epithelium. Afferent nerves are present, reaching into the superficial layers of the epithelium. With incompetence of the lower esophageal sphincter (LES), acid reflux results with consequently the possibility of pain and damage to the epithelium,
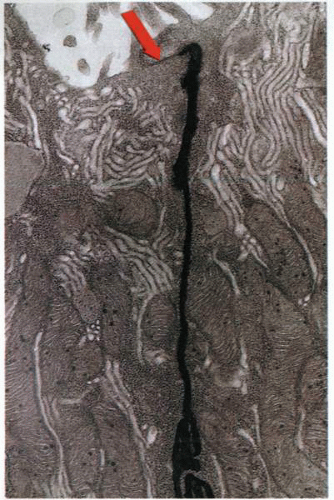
depending on the pH of the refluxate. Rather than being protected by a continuous tight junction, this epithelium has largely regional cell-to-cell contact via desmosomes. There is some evidence for the presence of tight junctions in the first layer of the epithelium, but the very few strands seen on EM freeze fracture show that these at best are “leaky” tight junctions. This epithelium, being multilayered, provides a winding path for proton diffusion into the deeper layers of the epithelium. It is significantly more acid sensitive than the gastric epithelium, which is provided with “tight” tight junctions.
depending on the pH of the refluxate. Rather than being protected by a continuous tight junction, this epithelium has largely regional cell-to-cell contact via desmosomes. There is some evidence for the presence of tight junctions in the first layer of the epithelium, but the very few strands seen on EM freeze fracture show that these at best are “leaky” tight junctions. This epithelium, being multilayered, provides a winding path for proton diffusion into the deeper layers of the epithelium. It is significantly more acid sensitive than the gastric epithelium, which is provided with “tight” tight junctions.
The thresholds of acidity for damage and pain may be different. A higher acidity is likely needed for damage to the epithelial cells compared to the acidity required for stimulation of the pain fibers. This is probably because the epithelial cells have acid-recovery mechanisms (such as Na+/H+ or anion exchange) mostly absent in the pain fibers. Also, pain fibers contain acid-sensitive ion channels (ASICs) that respond to environmental acidity. Depending on the location of the pain fibers and access thereto of H+, individuals have different sensitivities to luminal acidity. The therapeutic aims of acid control may vary, being elevation of mean diurnal pH for healing of erosions or prevention of acidic excursions for symptom relief, or both.
Esophageal healing
Given the absence of continuous tight junctions or only leaky tight junctions, the lower esophageal epithelium is an inviting target for acid back diffusion. Mucus is an aqueous gel and is unlikely to provide a significant barrier to acid. In water, protons can move faster than other cations due to their ability to jump from water molecule to water molecule, and there are continuous chains of water in aqueous gels.
Resistance to acid may therefore depend more on the ability of the esophageal epithelium to neutralize an acid load than on restriction of entry of acid between the cells. Neutralization of acid occurs at the surface due to net HCO3− secretion and also diffusion across the paracellular pathway, because
the epithelial cells can produce, buffer, or absorb H+



the epithelial cells can produce, buffer, or absorb H+
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree