Surgical Exposure of the Lower Extremity Arteries
Luke X. Zhan
Joseph L. Mills Sr.
DEFINITION
Chronic lower extremity ischemia, also known as peripheral artery disease (PAD), is a common condition managed by vascular specialists. The primary etiology is atherosclerosis. Atherosclerotic stenosis or occlusion of the peripheral arterial tree results in arterial insufficiency and end-organ (limb) ischemia. PAD is a major contributor to morbidity, reduced quality of life (QOL), and mortality in an increasing elderly demographic in the Western world.
DIFFERENTIAL DIAGNOSIS
The challenge for the vascular specialist is to determine whether the nature and severity of presenting symptoms correlate with the degree of chronic arterial insufficiency present or whether alternative etiologies, such as neuropathy, inflammation, infection, lymphatic or venous disease, and repetitive trauma, are more likely responsible. Definitive diagnosis is derived from detailed historic and physical examination findings correlated with appropriately directed noninvasive vascular laboratory and adjunctive imaging studies.
PATIENT HISTORY AND PHYSICAL FINDINGS
Patients with PAD may present with a spectrum of symptoms ranging in severity from none to varying degrees of claudication to severe or “critical” limb ischemia (“CLI” = ischemic rest pain, ulceration, and gangrene). Pulse palpation is an integral component of the physical examination. Femoral, popliteal, posterior tibial, and dorsal pedal pulses should be noted and graded (0 = absent; 1 = present but diminished; 2 = normal; 3 = enlarged, aneurysmal). Claudication is defined as muscular pain, cramping, aching, or discomfort in the lower limb, reproducibly elicited by exercise and relieved within 10 minutes of cessation. CLI has been traditionally defined as (1) persistent, recurring ischemic rest pain requiring opiate analgesia for more than 2 weeks and (2) ankle systolic pressure less than 50 mmHg or toe systolic pressure less than 30 mmHg (or absent pedal pulse in patients with diabetes).1 Ischemic rest pain typically is nocturnal, worsens with elevation, and is relieved by dependency. Pedal pulses are absent; dependent rubor, elevation pallor, and calf muscle atrophy are frequent accompaniments. CLI also includes ischemic foot ulceration and gangrene in the setting of ankle systolic pressure less than 50 to 70 mmHg or toe systolic pressure less than 40 mmHg in patients without diabetes (<50 mmHg in diabetics).
SURGICAL MANAGEMENT
Indication
All patients with PAD require comprehensive medical management and risk factor modification. Revascularization (either open bypass or endovascular intervention) is indicated in patients who remain symptomatic and significantly limited despite adequate risk factor modification, exercise, and medical management. The primary goal of intervention, in patients with lifestyle-limiting claudication, is to improve exercise tolerance and hence QOL. Patients with rest pain, tissue loss, and gangrene are at greater risk for limb loss and cardiovascular mortality (stroke, myocardial infarction) associated with systemic atherosclerosis than those who present with claudication alone. Revascularization in the CLI cohort is focused on wound healing and functional limb salvage as well as symptomatic relief and improvement in QOL.2,3
Preoperative Planning: Imaging and Risk Assessment/Mitigation
The vascular specialist must first determine, given the underlying disease burden, the severity of ischemic and infectious complications as well as the patient’s comorbidities, functional status, and anticipated longevity. Once it is decided that revascularization will improve the patient’s functional status and QOL, these same variables, in concert with anatomic assessment of the location, extent, and severity of occlusive arterial lesions will determine whether endovascular, open, or hybrid revascularization options are indicated. When bypass is selected as the preferred revascularization option, the goals of preoperative planning involve delineation of diseased arterial segment(s), identification of the most appropriate arterial inflow source, selection of the optimal bypass target for maximal outflow and target bed perfusion, and selection of the best available conduit. In practice, conduit availability is almost always a critical, rate-limiting factor because good quality, autogenous vein conduit is preferred in almost every circumstance.
Adequate preoperative planning depends on a thorough history and detailed physical examination.
The delineation of the relevant arterial anatomy on the index limb is facilitated by high quality, noninvasive vascular laboratory studies (ankle-brachial index and toe pressure measurements). These are supplemented by arterial color duplex ultrasound imaging. Arterial duplex is extremely accurate in the assessment of iliofemoral and femoropopliteal arterial occlusive disease but less so for infrageniculate (tibial-peroneal) lesions. Duplex enables differentiation of stenosis from occlusion and determination of lesion length and degree of calcification. Cross-sectional imaging studies such as computed tomography angiography (CTA) or magnetic resonance arteriography (MRA) may add complementary information, but most experienced operators prefer the precision and resolution inherent in catheter-based, intraarterial contrast arteriography for definitive preoperative planning, especially when bypass will be required to distal calf or pedal targets.
PAD is a coronary artery disease equivalent. Therefore, preoperative risk evaluation for overall cardiovascular-related mortality represents a component of preoperative planning.
In most patients with stable or minimally symptomatic coronary disease, preoperative risk-reduction efforts are best focused on optimizing medical management. Frequently, this includes statin and antiplatelet therapy, β-blockade, and optimization of hypertension management.
The surgical plan should be tailored to each patient’s needs based on extent of disease, conduit availability, and realistic long-term functional potential. Infrainguinal bypass may originate from the common, superficial, or deep femoral artery or the popliteal artery with a bypass target of the popliteal, tibial, or pedal/plantar arteries. The positioning, choice of incisions, and surgical techniques are dictated by type of bypass procedure deemed most appropriate under the circumstances.
TECHNIQUES
▪ Refer to references 4 through 9 for this section.
FEMORAL VESSEL EXPOSURE
Positioning
The patient is placed in supine position. A Foley catheter is inserted. Arms may be tucked to facilitate intraoperative prebypass and completion angiography.
Placement of Incision
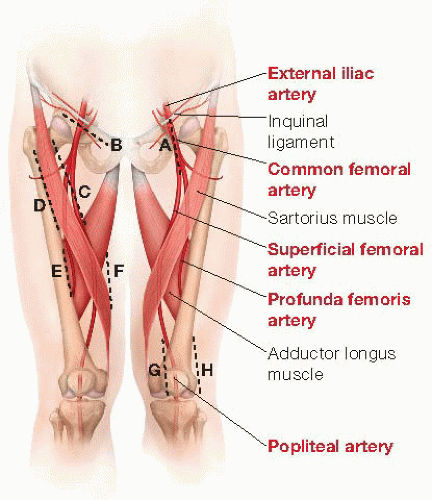
The common femoral artery (CFA) is located on a line between the pubic tubercle and anterior iliac spine, two fingerbreadths lateral to pubic tubercle. Palpation of the inguinal ligament and femoral pulse or direct arterial visualization with duplex imaging can localize the CFA bifurcation and guide optimal incision placement. Even when pulseless due to excessive calcification or occlusive disease, the CFA may be localized by reliance on anatomic landmarks and direct palpation, recognized as a firm tubular structure positioned within the femoral sheath.
The vertical groin incision is most commonly employed to provide optimal access to the entire length of the CFA. This should be created coaxially along the artery itself, continued from the inguinal ligament distally, and aimed at the medial aspect of the knee. The incision can be extended superiorly or inferiorly to increase arterial exposure as necessary to achieve optimal inflow (FIG 1, dashed line A).
Alternatively, especially in obese patients with substantial abdominal pannus, a curvilinear incision can be placed 1 cm below and parallel to the inguinal ligament to avoid potential skin maceration and wound complications that may accompany vertical incisions in this situation (FIG 1, dashed line B). Although the proximal superficial femoral and deep femoral arteries can be exposed via this incision, such a curvilinear or oblique incision limits further distal arterial exposure. It therefore would not be selected if an extensive common and deep femoral artery endarterectomy is anticipated as potentially necessary to optimize inflow.
The incision is carried sharply through the subcutaneous tissue and superficial fascia.
Dissection and Control of the Common, Superficial, and Proximal Deep Femoral Arteries
Deep to the subcutaneous tissue and superficial fascia, the dissection is extended longitudinally, even when using an oblique incision, to optimize the length of femoral exposure. Depending on the depth of dissection and subcutaneous adiposity, self-retaining Weitlaner or cerebellar retractors are carefully placed to optimize exposure while avoiding traction injury to femoral nerve branches or the common femoral vein. Further dissection through the femoral sheath exposes the anterior surface of the femoral artery.
The dissection plane should remain centered directly over the femoral artery. Encountering venous structures indicates medial deviation from the optimal plane; exposure of the iliopsoas muscle, femoral nerve fibers, or lymphatic vessels is an indication of lateral deviation. An increasing incidence of femoral incisional complications, including wound edge necrosis and separation, lymphatic leaks, femoral neuropraxia, and venous injuries are associated with incorrectly placed inguinal incisions for femoral exposure.
Dissect directly along the CFA both proximally and distally. Placement of silastic vessel loops around the
femoral artery and its larger branches aids in retraction, dissection, and mobilization.
Proximal dissection is continued along the CFA to the inguinal ligament. The inguinal ligament may be divided to aid in exposure or to enable extended endarterectomy. Caution is necessary in this area, as a prominent femoral vein tributary crosses anteriorly over the CFA in this area and is prone to injury if not identified, ligated, and divided early in the dissection. Inadvertent injury to this “vein of pain” produces retraction and troublesome bleeding. The medial and lateral femoral circumflex arteries, important collaterals in iliofemoral arterial occlusive disease, are identified at level of the inguinal ligament and individually controlled with removable clips or silastic vessel loops. Use of the former reduces clutter in the wound during endarterectomy or creation of the proximal anastomosis.
As the dissection proceeds distally, an abrupt change in caliber marks the femoral bifurcation and the origins of the deep (also known as “profunda femoris” in Latin) and superficial femoral arteries (SFA). The latter continues distally in the same plane; the former usually courses posteriorly and laterally away from the femoral bifurcation. After silastic loops are placed on each vessel, gentle upward traction on the CFA or SFA may help bring the deep femoral artery into view. The lateral circumflex iliac vein may course anteriorly over the origin of the deep femoral artery and should be ligated and divided to optimize exposure and control of the first segment of this vessel (FIG 2A).
Medial and distal dissection provides extended exposure of the proximal SFA (FIG 1, dashed line F). This vessel only occasionally has small branches in its proximal segment. A sensory branch of the femoral nerve may be present crossing the SFA from lateral to medial. Transection may result in medial thigh discomfort. Even extended femoral bifurcation dissections rarely require division of femoral nerve branches, which should be avoided to minimize postoperative paresthesias and dysesthesias.
 FIG 2 • A. Exposure of femoral vessels at groin. B. Anteromedial and posterolateral approaches to expose middle and distal segments of the superficial and deep femoral arteries. Incisions (C-F) correspond to FIG 1. |
Exposure of the Middle and Distal Segments of Deep Femoral Artery
Exposure of the distal portions of the deep femoral artery often enables use of shorter vein conduit in distal leg bypass or may improve outflow from proximal revascularization procedures (iliac angioplasty and stenting or aortofemoral bypass). These segments are easily exposed from either posteromedial or anteromedial approaches (FIG 1, dashed line C-F). The approach should be dictated by the indication (inflow sources or outflow target); an additional consideration is the necessity to obtain exposure in a native field, either in the setting of prior dissection or femoral graft infection.
Incisions are placed along either the medial (anteromedial approach; dashed line C and F in FIG 1) or lateral borders (posterolateral approach) of the sartorius muscle (FIG 1, dashed line D and E). The dissection plane is developed through the subcutaneous tissue and fascia, passing lateral or medial to the sartorius, respectively. Mobilize and retract sartorius muscle laterally or medially, depending on approach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree