KEY POINTS
The human spleen plays a key immunologic role in defense against a number of organisms, particularly encapsulated bacteria.
The spleen can cause significant morbidity and/or hematologic disturbance if it becomes hyperfunctioning (hypersplenism) or hypertrophied (splenomegaly).
There is a broad spectrum of nontraumatic diseases for which elective splenectomy can be curative or palliative. They can be broadly categorized as red blood cell disorders and hemoglobinopathies, white blood cell disorders, platelet disorders, bone marrow disorders, infections and abscesses, cysts and tumors, storage diseases and infiltrative disorders, and miscellaneous conditions.
Partial splenectomy may be a suitable alternative to total splenectomy for certain conditions of hypersplenism or splenomegaly, particularly in children in whom preservation of splenic immunologic function is especially important.
Preoperative splenic artery embolization for elective splenectomy has benefits and disadvantages. It may be most suitable in cases of enlarged spleen. Conclusive evidence is lacking.
Laparoscopic splenectomy provides equal hematologic outcomes with decreased morbidity compared with the open operation. The laparoscopic approach has emerged as the standard for elective, nontraumatic splenectomy.
Inadvertent intraoperative splenic injury is a scenario for which every abdominal surgeon should be prepared. Availability of a predetermined algorithm, with emphasis on the patient’s condition, facilitates intraoperative decision making.
Overwhelming postsplenectomy infection (OPSI) is an uncommon but potentially grave disease. Children and those undergoing splenectomy for hematologic malignancy are at elevated risk.
Antibiotic prophylactic strategies against OPSI vary widely. Data regarding their use are lacking.
Vaccination of the splenectomized patient remains the most effective prevention strategy against OPSI. Preoperative vaccination before elective splenectomy is most prudent.
HISTORICAL BACKGROUND
For more than two millennia, the spleen has perhaps been the least understood and most underappreciated major organ. The central role played by the spleen in regulating the immune system as well as influencing metabolic and endocrine functions has only become clear over the past few decades. Our previous notion of the spleen as an utterly dispensable filter of the blood has been dispelled, enlightening our surgical approach to this fascinating organ.
Many of the “founding fathers of medicine” have weighed in on the anatomy and function of the spleen over the centuries. Hippocrates1,2,3 in the fourth century bc was one of the first to write on the spleen. He taught broadly on the need for balance and equilibrium between the patient and his environment. Illness arose from disharmony in nature, particularly among the patient’s four humors: blood, phlegm, black bile (melancholia), and yellow bile. Hippocrates wrote of a direct connection between the brain and spleen and its particular association with the black bile. These ideas would influence thinking about the role of the spleen for more than 1000 years.2
Aristotle, later in the same era, famously stated that, “Nature makes nothing in vain,” yet held the spleen to be an organ of minor importance whose main role was to counterbalance the liver.4 He also described how the “hot character” of the spleen aided in digestion.
Galen, in the second century ad, engaged in more serious anatomic investigation espousing the early belief that function followed structure. His investigations, though pioneering, lacked sufficient rigor, evidenced by his contention that black bile or melancholia flowed from the liver to the spleen and then through the short gastric vessels into the stomach to be excreted. The influence of Galen’s teaching endured for more than 1200 years.
In the early seventeenth century, several physician scientists, Malpighi being the most prominent, began testing hypotheses on splenic function by splenectomizing dogs. He reportedly followed several dogs 5 years postoperatively, noting their healthy survival though apparent ravenous hunger and enhanced sexual appetites. The spleen, still in the era of “balanced humors,” was thus felt to play a role in balancing various appetites as well. In addition to melancholy, the spleen became associated with anger and, paradoxically, was also seen as the “seat of laughter.”5
The claim for the first human splenectomy may have predated that of canine splenectomy. Andriano Zaccavello was credited in 1549 with having performed a splenectomy on a middle-aged woman. This claim remains shrouded in controversy, and the indication for the surgery and whether in fact splenectomy was performed have been called into question. The patient apparently survived. Most patients who underwent splenectomy in the three centuries that followed fared badly. The vast majority of splenectomies performed were partial. Most of these patients required surgery for left upper quadrant stab wounds sustained in battles or duels resulting in partial or complete splenic prolapse.6
It was in the early eighteenth century that the growing body of anatomic microstructural knowledge began to turn the tide on the long-held theory of health and disease deriving from a balance of the four humors. William Henson believed the spleen to be a ductless vascular gland similar to the thyroid and adrenals. In 1777, he wrote of the lymphatic nature of the spleen and its filtering function and even suggested its role in hematopoiesis.13
Rudolf Virchow, one of the first to discover leukemia, implicated the spleen as figuring prominently in all leukemia patients. He suspected that the spleen was responsible for generating the leukocytes in large quantities in these patients. There soon followed an enthusiastic effort by surgeons to cure leukemia by splenectomy. Dr. Thomas Bryant performed the first splenectomy in 1866 in a patient with leukemia. The patient died, as did all 14 patients who underwent splenectomy for leukemia over the next 15 years. After his second consecutive mortality in this setting, Bryant declared that “the operation is physiologically unsound & surgically unsafe for leukemia and should not be performed.”7 In 1908, Johnson reported a series of 99 splenectomies for leukemia with an 85% mortality rate. Unfortunately, it took several decades for his words to be heeded.
In 1916, a medical student from Prague named Paul Kaznelson wrote on the key role played by the spleen in the destruction of platelets leading to the first reported (and successful) splenectomy for a patient with idiopathic thrombocytopenia purpura.2
As surgeons’ experience with the procedure grew, the associated morbidity and mortality decreased. By 1920, the Mayo Clinic experience with splenectomy reported a reduced mortality rate of 11%.1
O’Donnell in 1929 was the first to describe fatal postsplenectomy sepsis in a child who had undergone the surgery for hemolytic anemia.3 It took Springer’s 1973 review of almost 2800 postsplenectomy patients and the 2.5% incidence of sepsis-induced mortality (vs. 0.01% in the general population) to reorient surgeons to more conservative splenic procedures.2,3
The advent of minimally invasive surgery and laparoscopic splenectomy in the early 1990s represented a clear advance, benefitting the patient through this evolution of surgical technique. Most large series of laparoscopic splenectomy for benign and malignant indication now report a mortality rate of <1%.9,10 As even more contemporary research reveals the spleen to play a central role in immune, metabolic, and endocrine function, it follows that the surgeon’s role going forward will be to preserve this organ and its functions whenever possible.
EMBRYOLOGY AND ANATOMY
Consisting of an encapsulated mass of vascular and lymphoid tissue, the spleen is the largest reticuloendothelial organ in the body. Arising from the primitive mesoderm as an outgrowth of the left side of the dorsal mesogastrium, by the fifth week of gestation, the spleen is evident in an embryo 8 mm long.
Development begins through the formation of the splanchnic mesodermal plate, derived from the mesoderm, at embryonic day 12. The embryonic spleen is first colonized by erythroid and myeloid progenitor cells at 2 weeks of gestation. Following soon thereafter, the hematopoietic stem cells take up residence in the forming spleen.11 The spleen assumes an important hematopoietic role until the fifth month of gestation. After birth, splenic erythropoietic function may persist in some hematologic disorders.12
The organ continues its differentiation and migration to the left upper quadrant, where it comes to rest with its smooth, diaphragmatic surface facing posterosuperiorly.8
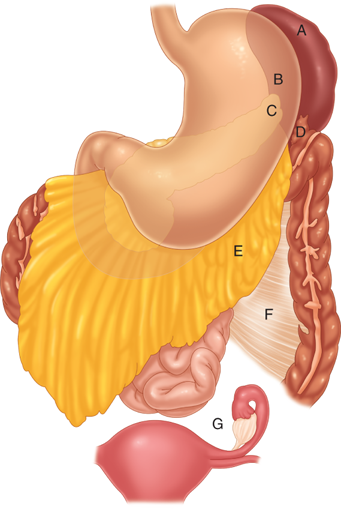
The most common anomaly of splenic embryology is the accessory spleen. Present in up to 20% of the population, one or more accessory spleens may also occur in up to 30% of patients with hematologic disease. Over 80% of accessory spleens are found in the region of the splenic hilum and vascular pedicle. Other locations for accessory spleens in descending order of frequency are the gastrocolic ligament, the pancreas tail, the greater omentum, the stomach’s greater curve, the splenocolic ligament, the small and large bowel mesentery, the left broad ligament in women, and the left spermatic cord in men (Fig. 34-1).8,13
The abdominal surface of the diaphragm separates the spleen from the lower left lung and pleura and the ninth to eleventh ribs. The visceral surface faces the abdominal cavity and contains gastric, colic, renal, and pancreatic impressions. Spleen size and weight vary with age, with both diminishing in the elderly and in those with underlying pathologic conditions. The average adult spleen is 7 to 11 cm in length and weighs 150 g (range, 70 to 250 g).
The spleen’s superior border separates the diaphragmatic surface from the gastric impression of the visceral surface and often contains one or two notches, which are particularly pronounced when the spleen is greatly enlarged.
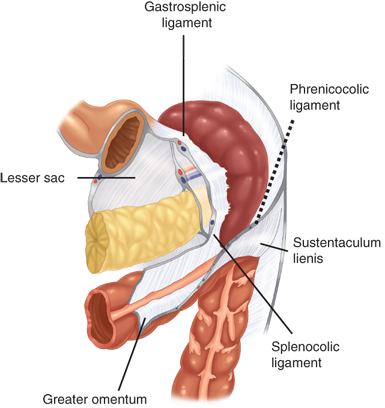
Of particular clinical relevance, the spleen is suspended in position by several ligaments and peritoneal folds to the colon (splenocolic ligament), the stomach (gastrosplenic ligament), the diaphragm (phrenosplenic ligament), and the kidney, adrenal gland, and tail of the pancreas (splenorenal ligament) (Fig. 34-2). The gastrosplenic ligament contains the short gastric vessels; the remaining ligaments are avascular, with rare exceptions, such as in patients with portal hypertension. The relationship of the pancreas to the spleen also has important clinical implications. In cadaveric anatomic series, the tail of the pancreas has been demonstrated to lie within 1 cm of the splenic hilum 75% of the time and to actually abut the spleen in 30% of patients.2
The spleen derives most of its blood from the splenic artery, the longest and most tortuous of the three main branches of the celiac artery. The splenic artery can be characterized by the pattern of its terminal branches. The distributed type of splenic artery is the most common (70%) and is distinguished by a short trunk with many long branches entering over three-fourths of the spleen’s medial surface. The less common magistral type of splenic artery (30%) has a long main trunk dividing near the hilum into short terminal branches, and these enter over 25% to 30% of the spleen’s medial surface. The spleen also receives some of its blood supply from the short gastric vessels that branch from the left gastroepiploic artery running within the gastrosplenic ligament. The splenic vein joins the superior mesenteric vein to form the portal vein and accommodates the major venous drainage of the spleen.
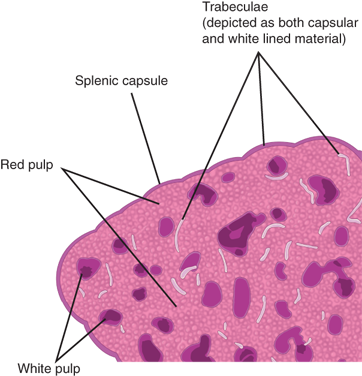
When a normal, freshly excised spleen is sectioned, the cut surface is finely granular and predominantly dark red with whitish nodules distributed liberally across its expanse. This gross observation reflects the spleen’s microstructure. The splenic parenchyma is composed of two main elements: the red pulp, constituting approximately 75% of total splenic volume, and the white pulp (Fig. 34-3). At the interface between the red and white pulp is the narrow marginal zone.
Blood enters the red pulp through cords comprised of fibroblasts and reticular fibers, which contain many macrophages and lack an endothelial lining. The blood then passes from these “open” cords to venous sinuses, which are surrounded and separated by the same reticulum, and ultimately drains into tributaries of the splenic vein. An understanding of the microanatomy of these sinuses has elucidated the mechanical filtration function of the spleen. Unlike the cords of the red pulp, the sinuses of the red pulp are lined by endothelial cells. These cells contain unique stress fibers that connect the endothelial cells and that contain actin and myosin–like filaments capable of producing a sliding action. When activated, these filaments can create slits or gaps between the endothelial cells through which blood can then pass from the cords.11 Aging erythrocytes with stiffer membranes get stuck trying to pass into the sinus and are phagocytized by macrophages within the red pulp.12
The red pulp thus serves as a dynamic filtration system, enabling macrophages to remove microorganisms, cellular debris, antigen-antibody complexes, and senescent erythrocytes from the circulation.
Around the terminal millimeters of splenic arterioles, a periarticular lymphatic sheath replaces the native adventitia of the vessel. The sheath is comprised of T lymphocytes and intermittent aggregations of B lymphocytes or lymphoid follicles. When antigenically stimulated, the follicles, serving as centers of lymphocyte proliferation, develop germinal centers, which regress as the stimulus or infection subsides. This white pulp consists of nodules that normally are ≤1 mm in size but can increase to several centimeters when nodules coalesce, as occurs in certain lymphoproliferative disorders. At the junction between the white and red pulp is the marginal zone, where lymphocytes are more loosely aggregated.
As well as serving as a transit area, the marginal zone is home to its own unique population of cells. Notably two specific types of macrophages reside there, marginal zone macrophages and marginal zone metallophilic macrophages. The former play an important role in the targeting and clearance of certain bacterial pathogens. The latter have been shown to be the main producers of interferons A and B in response to a viral challenge.11
PHYSIOLOGY AND PATHOPHYSIOLOGY
The spleen is contained by a 1- to 2-mm thick capsule. In humans, the capsule is rich in collagen and contains some elastin fibers. Many mammals have splenic capsules and trabeculae with abundant smooth muscle cells, which upon autonomic stimulation contract to expel large volumes of stored blood into the general circulation. The human splenic capsule and trabeculae, by contrast, contain few or no smooth muscle cells.
Total splenic inflow of blood is approximately 250 to 300 mL/min. Blood flows through successively tapering arteries to arterioles, traverses the white pulp, crosses the marginal zone, and enters the red pulp. From that entry, the flow rate through the spleen may vary greatly. Animal studies measuring the transit times of isotopically labeled blood through the spleen have revealed three distinct velocities of flow. Humans have long been recognized to have both a fast or closed circulation—with blood passing directly from arterioles into venous sinuses—and a slower or open circulation. Most of the spleen’s filtration function occurs via the slower circulation. During open circulation, blood percolates through the reticular space and splenic cords, thus gaining access through gaps or slits in the endothelial cell lining to the sinuses as previously described. Flowing into and out of the venous sinuses through these gaps, the blood is exposed to extensive contact with splenic macrophages. These are responsible for the innate immune response of the spleen, which occurs largely within the marginal zone. The white pulp, by contrast, is involved only in adaptive immunity. In addition, because the passage of plasma through these spaces does not slow in a similar manner, a temporary and unique adhesive contact between blood cells and components of the splenic cord may occur. That there is a selective slowing of blood cell flow versus plasma flow is further evidenced by the fact that within the spleen, the erythrocyte concentration (hematocrit) is twice that of the general circulation. During this contact with splenic macrophages, it is likely that the removal of both cellular debris and senescent blood cells occurs.14
The process by which the spleen removes erythrocyte inclusions, such as Heinz bodies (intracellular altered hemoglobin), without cell lysis while red blood cells travel through the spleen is not well understood. The spleen acts as the major site for clearance from the blood of damaged or aged red blood cells and, in addition, has a part in the removal of abnormal white blood cells and platelets. A minimum of 2 days of the erythrocyte’s 120-day life cycle is spent sequestered in the spleen. Daily, approximately 20 mL of aged red blood cells are removed. Evidence suggests that, as erythrocytes age, previously undetected antigens on their surfaces may attach to autoantibodies in the circulation; then macrophages may bind to the antibodies and initiate phagocytosis. It is probable that the erythrocyte is damaged over time by multiple passages through the spleen as well as delayed transit through the congested and relatively hypoxic and acidotic environment of the splenic cords.
The spleen can also serve as an extra medullary site for hematopoiesis, if required. Another role played by the spleen is in recycling iron. Erythrocytes in large numbers are destroyed intravascularly throughout the body. The released hemoglobin is then bound to haptoglobin, which is ultimately scavenged from the circulation in the spleen.15
The spleen plays a vital, although not indispensable, role in host defense evidenced by the healthy survival of splenectomized patients. Both innate and adaptive immune responses (historically categorized as cell-mediated and humoral immunity) occur within the spleen.
In addition to the previously noted activities of the marginal zone macrophages, marginal zone B cells serve to detect circulating pathogens and respond quickly to either differentiate into immunoglobulin M (IgM)–producing plasma cells or to function as antigen-presenting cells (APCs), which facilitate pathogen removal and destruction.
It is APC entry in the white pulp in particular that is key to the initiation of the adaptive immune response. Antigens are thus presented to immunocompetent centers within the lymphoid follicles. This gives rise to the elaboration of immunoglobulins (predominantly IgM). After an antigen challenge, such an acute IgM response results in the release of opsonic antibodies from the white pulp of the spleen. Antigen clearance is then facilitated by the splenic and hepatic reticuloendothelial systems.
The structure and immunophysiology of the white pulp is very similar to that of lymph nodes, with the notable difference being that material enters the lymph node in the lymph whereas it is delivered to the white pulp in the blood.16
The spleen also produces opsonins, tuftsin, and properdin. Circulating monocytes are converted within the red pulp into fixed macrophages that account for the spleen’s remarkable phagocytic activity.
The spleen also appears to be a major source of the protein properdin, important in the initiation of the alternate pathway of complement activation. The splenic reticuloendothelial system is better able to clear bacteria that are poorly or inadequately opsonized from the circulation than is the hepatic reticuloendothelial system. Encapsulated bacteria generally fit such a profile, hence the risk posed by pneumococcus and Haemophilus influenzae to an asplenic patient. There appears to be sufficient physiologic capacity within the complement cascade to withstand the loss of tuftsin and properdin production without an increase in patient vulnerability after splenectomy.17,18,19
In patients with chronic hemolytic disorders, splenic tissue may become permanently hypertrophied. The reticular spaces of the red pulp become distended with macrophages engorged with the products of erythrocyte breakdown, and splenomegaly can result. It is important to distinguish between splenomegaly and hypersplenism, two similar but distinct terms that are critical to understand when discussing splenic pathology. Splenomegaly refers simply to abnormal enlargement of the spleen. Splenomegaly is described variably within the surgical literature as moderate, massive, and hyper, which reflects a lack of consensus. Most would agree, however, that splenomegaly applies to organs weighing ≥500 g and/or averaging ≥15 cm in length.
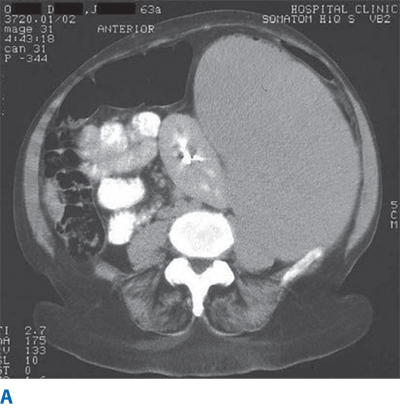
Massive splenomegaly similarly lacks a consensus definition but has been described variably as spleens >1 kg in mass or >22 cm in length (Fig. 34-4).6 Spleens palpable below the left costal margin are thought to be at least double normal size, with an estimated weight of ≥750 g.20
There is not a single, universally accepted standard, but most would agree that an ex vivo mass of >1 kg or a pole-to-pole length of >15 cm generally qualifies as splenomegaly. Hypersplenism often is found in association with splenomegaly but is not synonymous with it. Hypersplenism is defined as the presence of one or more cytopenias in the context of a normally functioning bone marrow.
Disorders causing hypersplenism can be categorized as either (a) those in which increased destruction of abnormal blood cells occurs in an intrinsically normal spleen (e.g., hemolytic anemias) or (b) primary disorders of the spleen resulting in increased sequestration and destruction of normal blood cells (e.g., infiltrative disorders).
The life cycles of cellular elements vary widely in human blood. A neutrophil in circulation has a normal half-life of approximately 6 hours. The spleen’s role in the normal clearance of neutrophils is not well established. It is clear that hypersplenism may result in neutropenia through sequestration of normal white blood cells or the removal of abnormal ones. Platelets, on the other hand, generally survive in the circulation for 10 days. Under normal circumstances, a third of the total platelet pool is sequestered in the spleen. Thrombocytopenia may result from excessive sequestration of platelets as well as accelerated platelet destruction in the spleen. Splenomegaly may result in sequestration of up to 80% of the platelet pool. The spleen may also contribute to the immunologic alteration of platelets, which leads to thrombocytopenia in the absence of splenomegaly (e.g., idiopathic thrombocytopenic purpura [ITP]).21
The immunologic functions of the spleen are consistent with those of other lymphoid organs. It is a site of bloodborne antigen presentation and the initiation of T- and B-lymphocyte activities involved in humoral and cellular immune responses. Alteration of splenic immune function often gives rise to antibody production, which results in blood cell destruction.
Although the spleen contributes to the process of erythrocyte maturation, in adult humans there is little evidence of normal hematopoietic function. The spleen does have a minor role in hematopoiesis in the fourth month in the human fetus, and reactivation can occur in childhood if the bone marrow fails to meet the hematologic needs. Splenic hematopoiesis giving rise to abnormal red blood cells is seen in adults with myeloproliferative disorders. In addition, in response to some anemias, elements of the red pulp may revert to hematopoiesis.
IMAGING FOR EVALUATION OF SIZE AND PATHOLOGY
Prior to operation, thorough assessment of anatomic detail and functional status of the spleen is essential for surgical planning. Special preoperative consideration needs to be given to patients with splenomegaly because minimally invasive methods increase in difficulty with increasing size of the spleen. Other indications for splenic imaging include trauma, investigations of left upper quadrant pain, characterization of splenic lesions such as tumors, cysts, and abscesses, and guidance for percutaneous procedures.22,23
Preoperative imaging of the spleen is primarily performed to obtain an accurate assessment of the splenic volume in order to confirm and document splenomegaly as well as to exclude any large splenic lesion that could affect the surgical resection plane. Identification of the presence of accessory spleens in the preoperative setting is also important, although lack of accessory tissue on the imaging should not preclude a thorough intraoperative search.
The guidelines of the European Association for Endoscopic Surgery suggest that all patients should undergo preoperative imaging.20 Some of the common imaging modalities include ultrasound and computed tomography (CT), both enabling measurement of splenic size and volume. When desired, the splenic volume may be calculated using a formula for the volume of a prolate ellipsoid: Volume (cc) = Length (cm) × Width (cm) × Height (cm) × 0.52.43 Other imaging modalities, although not as commonly used, include nuclear medicine studies and magnetic resonance imaging (MRI).
Ultrasound is the least invasive mode of splenic imaging. It is rapid, easy to perform, and does not expose the patient to ionizing radiation. It is often the first imaging modality applied to the spleen during evaluation and resuscitation of the trauma patient, although questions of sensitivity and specificity remain.24,25 In the elective setting, such as for routine diagnostic purposes or for preoperative planning, it is the least costly modality available, and the sensitivity of ultrasound for detecting textural lesions of the spleen can be quite good in experienced hands.26
When examining a normal spleen, differentiation between red and white pulp is not possible, and a homogeneous acoustic echotexture should be visualized. Splenic artery and vein patency may be assessed using Doppler imaging. Splenic artery anatomy has been classified commonly into two patterns: distributed and magistral. This variability in vascular distribution visualized by Doppler imaging becomes important when there is consideration of performing a partial splenectomy.
Percutaneous ultrasound-guided procedures for splenic disease (e.g., cyst aspiration, biopsy), historically avoided due to the risk of hemorrhage and other complications, are becoming more common as the safety of these procedures is being increasingly demonstrated.22,23
CT affords a high degree of resolution and detail of the splenic parenchyma, vasculature, and its relationship to neighboring structures. Modern-day CT is more automated and thus less operator dependent than ultrasound, which makes it the preferred imaging modality for many practitioners. Furthermore, CT has become an invaluable tool in the evaluation and management of the blunt trauma patient, and standardized scoring systems for splenic trauma based on CT images now aid in management decisions.27 In the nontrauma setting, CT is extremely useful for assessment of splenomegaly, identification of solid and cystic lesions, and guidance of percutaneous procedures.3 The use of iodinated contrast material adds diagnostic clarity to CT imaging of the spleen, although at the cost of the small but real risks of renal impairment or allergic reaction. Three-dimensional reconstruction after CT scan may help to predict the difficulty of the procedure and to choose the best surgical approach: open or laparoscopic.29,30
The appearance of normal splenic tissue on a noncontrast CT is uniform parenchymal attenuation. On a contrast-enhanced CT, the appearance of the spleen depends largely on the timing of the intravenous bolus administration of contrast material. Due to the different rates of flow through the red and white pulp, the spleen appears heterogeneously enhancing during the first minute after initiation of intravenous administration of contrast material. The frequency of these artifacts increases with advancing patient age. When evaluating a splenic abscess, a contrast-enhanced CT should be considered.28,43
Three-dimensional CT volumetry is a novel modality that measures the volume of the spleen. This tool may be of some benefit in planning technically challenging cases involving splenomegaly. A recent retrospective review of laparoscopic splenectomy patients who underwent CT volumetry preoperatively demonstrated a higher conversion rate when the spleen was measured to be greater than 2700 cc.29 This information may aid in preoperative planning in determining which patients may benefit from a hand-assisted laparoscopic surgery approach or open approach.97
Rarely is plain radiography used for primary splenic imaging. Plain films can indirectly provide an outline of the spleen in the left upper quadrant or suggest splenomegaly by revealing displacement of adjacent air-filled structures (e.g., the stomach or splenic flexure of the colon). Plain films may also demonstrate splenic calcifications. Splenic calcifications often are found in association with splenomegaly but are otherwise a nonspecific finding. Splenic calcifications can indicate a number of benign, neoplastic, or infectious processes, including phlebolith, splenic artery aneurysm, sickle cell changes, tumors (e.g., hemangioma, hemangiosarcoma, lymphoma), echinococcosis, or tuberculosis.26
Although MRI offers excellent detail and versatility in abdominal imaging, it is more expensive than CT scanning or ultrasound and offers no obvious advantage for primary imaging of the spleen. MRI can be a valuable adjunct to the more commonly used imaging techniques when splenic disease is suspected but not definitively diagnosed.26,28
Magnetic resonance (MR) signal characteristics of the spleen are related to the relative ratio of red and white pulp and the relationship of the timing of the intravenous (IV) contrast bolus and the time of image acquisition. The spleen will generally have a homogeneous MR signal on noncontrast images. On contrast MRI, the spleen appears to have heterogeneous enhancement during the arterial phase of contrast enhancement.
Angiography of the spleen most commonly refers to invasive arterial imaging, and when it is combined with therapeutic splenic arterial embolization (SAE), there are multiple applications for this procedure: localization and treatment of hemorrhage in select trauma patients31; delivery of a variety of therapies in patients with cirrhosis or portal and sinistral hypertension and in transplant patients32; and adjunct (or, more controversially, as an alternative) to splenectomy for treatment of hematologic disorders such as ITP or hypersplenism.33 Preoperative or intraoperative SAE for elective splenectomy is also a common, although not universal, practice. Few prospective data have been published in the last 5 years on preoperative SAE.34 Preoperative SAE is purported not only to facilitate operation but also possibly to allow a laparoscopic approach in patients whose spleens had previously been considered too large for, or otherwise not amenable to, laparoscopic resection. Limited success in using partial SAE as an alternative to therapeutic splenectomy in chronic ITP has been previously reported.35 Its detractors argue that the need for increased analgesics and occasional extended hospital stay preoperatively, the possibility of pancreatitis, and the well-described risks of invasive arteriography negate any presumed benefits of preoperative SAE.
Radioscintigraphy with technetium-99m sulfur colloid demonstrates splenic location and size. It may be especially helpful in locating accessory spleens after unsuccessful splenectomy for ITP and has recently proven useful in diagnosing splenosis.36,37 Unfortunately, no conclusive outcome benefit has been shown for preoperative technetium scanning before splenectomy.38,39 When dealing with diseases of platelet sequestration, indium-labeled autologous platelet scanning (ILAPS) demonstrates whether platelet sequestration is predominantly in the spleen, liver, or both. This becomes important in deciding whether or not a patient will benefit from a splenectomy. ILAPS is a nuclear imaging modality in which autologous platelets are reinfused into the patient after ex vivo labeling. Subsequent scintigraphy demonstrates the site(s) of platelet sequestration and clearance. It has been proposed that patients with purely or predominantly splenic sequestration determined by ILAPS may be more likely to respond to splenectomy than those exhibiting hepatic, mixed, or diffuse patterns.33
An emerging and novel application for spleen scintigraphy may be as a noninvasive method to diagnose nonalcoholic steatohepatitis (NASH). NASH, which may lead to cirrhosis, can result from nonalcoholic fatty liver disease (NAFLD), the most common cause of steatosis. With the rising prevalence of obesity in the United States, NAFLD is also increasingly prevalent.12 The diagnosis of progression from NAFLD to NASH has been dependent on histologic assessment of tissue obtained from liver biopsy. Characteristics unique to NASH have been reported, among them the association of splenic enlargement, not seen to a similar degree in NAFLD.10
In addition, the ratio of liver-to-spleen uptake determined by scintigraphy has been found to be predictably altered in NASH patients. The liver-to-spleen uptake ratio is significantly decreased in NASH patients, but not NAFLD patients, leading some to conclude that technetium-99m-phytate scintigraphy is a reliable tool to differentiate NASH from NAFLD.40
INDICATIONS FOR SPLENECTOMY
Splenectomy is therapeutic for a large host of conditions, which can be divided into the following broad categories: (a) benign, including red blood cell disorders, hemoglobinopathies, and platelet disorders; (b) malignant, including white blood cell disorders and bone marrow disorders (myeloproliferative disorders); and (c) other, including cysts and tumors, infections and abscesses, splenic rupture, and a number of miscellaneous disorders and lesions (Table 34-1).
| DISEASE/CONDITION | INDICATIONS FOR SPLENECTOMY | RESPONSE TO SPLENECTOMY |
|---|---|---|
| Hereditary spherocytosis | Hemolytic anemia, recurrent transfusions, intractable leg ulcers | Improves or eliminates anemia |
| Hereditary elliptocytosis | Limited role for splenectomy | — |
| Pyruvate kinase deficiency | Only in severe cases, recurrent transfusions | Decreased transfusion requirement, palliative only |
| Glucose-6-phosphate dehydrogenase deficiency | None | — |
| Warm-antibody autoimmune hemolytic anemia | Failure of medical (steroid) therapy | 60%–80% response rate, recurrences common |
| Sickle cell disease | History of acute sequestration crisis, splenic symptoms, or infarction (consider concomitant cholecystectomy) | Palliative, variable response |
| Thalassemia | Excessive transfusion requirements, symptomatic splenomegaly, or infarction | Diminished transfusion requirements, relief of symptoms |
| Acute myeloid leukemia (AML) | Intolerable symptomatic splenomegaly | Relief of abdominal pain and early satiety |
| Chronic myeloid leukemia | Symptomatic splenomegaly | Relief of abdominal pain and early satiety |
| Chronic myelomonocytic leukemia | Symptomatic splenomegaly | Relief of abdominal pain and early satiety |
| Essential thrombocythemia | Only for advanced disease (i.e., transformation to myeloid metaplasia or AML) with severe symptomatic splenomegaly | Relief of abdominal pain and early satiety |
| Polycythemia vera | Only for advanced disease (i.e., transformation to myeloid metaplasia or AML) with severe symptomatic splenomegaly | Relief of abdominal pain and early satiety |
| Myelofibrosis (agnogenic myeloid metaplasia) | Severe symptomatic splenomegaly | 76% clinical response at 1 y, high risk of hemorrhagic, thrombotic, and infectious complications (26%) |
| Chronic lymphocytic leukemia | Cytopenias and anemia | 75% response rate |
| Hodgkin’s disease | Surgical staging in selected cases | — |
| Non-Hodgkin’s lymphoma | Cytopenias, symptomatic splenomegaly | Improved complete blood count values, relief of symptoms |
| Idiopathic thrombocytopenic purpura | Failure of medical therapy, recurrent disease | 75%–85% rate of long-term response |
| Thrombotic thrombocytopenic purpura | Excessive plasma exchange requirement | Typically curative |
| Abscesses of the spleen | Therapy of choice | Curative |
| Symptomatic parasitic cysts | Therapy of choice | Curative; exercise caution not to spill cyst contents |
| Symptomatic nonparasitic cysts | Partial splenectomy for small cysts; unroofing for large cysts | Curative |
| Gaucher’s disease | Hypersplenism | Improves cytopenias; does not correct underlying disease |
| Niemann-Pick disease | Symptomatic splenomegaly | Improves symptoms; does not correct underlying disease |
| Amyloidosis | Symptomatic splenomegaly | Improves symptoms; does not correct underlying disease |
| Sarcoidosis | Hypersplenism or symptomatic splenomegaly | Improves symptoms and cytopenias; does not correct underlying disease |
| Felty’s syndrome | Neutropenia | 80% durable response rate |
| Splenic artery aneurysm | Splenectomy best for distal lesions near splenic hilum | Curative |
| Portal hypertension | Portal or sinistral hypertension due to splenic vein thrombosis | Palliative |
Overall, the most common indication for splenectomy is trauma to the spleen, whether external trauma (blunt or penetrating) or iatrogenic injury (e.g., during operative procedures for other reasons). Inadvertent intraoperative injury to the spleen in the nontrauma patient is discussed in a later section. Management of splenic injury in the trauma patient is beyond the scope of this chapter. The most common indication for elective splenectomy is ITP.
Hereditary spherocytosis (HS) results from an inherited dysfunction or deficiency in one of the erythrocyte membrane proteins (spectrin, ankyrin, band 3 protein, or protein 4.2). The resulting destabilization of the membrane lipid bilayer allows a pathologic release of membrane lipids. The red blood cell assumes a more spherical, less deformable shape, and the spherocytic erythrocytes are sequestered and destroyed in the spleen. Hemolytic anemia ensues; in fact, HS is the most common hemolytic anemia for which splenectomy is indicated. HS is inherited primarily in an autosomal dominant fashion; the estimated prevalence in Western populations is 1 in 5000.4142,43
Patients with typical HS forms may have mild jaundice. Splenomegaly usually is present on physical examination. Laboratory examination reveals varying degrees of anemia: patients with mild forms of the disease may have no anemia; patients with severe forms may have hemoglobin levels as low as 4 to 6 g/dL. The mean corpuscular volume is typically low to normal or slightly decreased. For screening, a combined elevated mean corpuscular hemoglobin concentration and an elevated erythrocyte distribution width are an excellent predictor. Other laboratory indicators of HS include those providing evidence of rapid red blood cell destruction, including elevated reticulocyte count, elevated lactate dehydrogenase level, and increased level of unconjugated bilirubin. Spherocytes are readily apparent on peripheral blood film.
Risks and benefits should be assessed carefully before splenectomy and cholecystectomy are performed for HS.41 The main indications are symptomatic hemolytic anemia, growth retardation, skeletal changes, leg ulcers, and extramedullary hemopoietic tumors in young patients. If gallstones coexist with spherocytosis, the gallbladder should be removed, but prophylactic cholecystectomy without gallstones is not recommended. Near total splenectomy is advocated in children. Dramatic clinical improvement—despite persistent hemolysis—usually occurs after splenectomy in patients with severe disease. Because children can be affected with HS, the timing of splenectomy is important and is aimed at reducing the quite small possibility of overwhelming postsplenectomy sepsis. Delaying such an operation until the patient is between the ages of 4 and 6—unless the anemia and hemolysis accelerate—is recommended by most experts.44
Hereditary elliptocytosis (HE) merits brief discussion to distinguish it from HS. Both HS and HE are conditions of the red blood cell membrane that result from genetic defects in skeletal membrane proteins. With the HE defect, the red blood cell elongates as it circulates, so that far fewer red blood cells are sequestered or destroyed when transiting the splenic parenchyma. Unless more than 50% of red blood cells are affected (a scenario that could permit development of a clinical syndrome like HS), HE may be considered harmless.
Red blood cell enzyme deficiencies associated with hemolytic anemia may be classified into two groups: deficiencies of enzymes involved in glycolytic pathways, such as pyruvate kinase (PK) deficiency, and deficiencies of enzymes needed to maintain a high ratio of reduced to oxidized glutathione in the red blood cell, protecting it from oxidative damage, such as glucose-6-phosphate dehydrogenase (G6PD) deficiency.45
The most common red blood cell enzyme deficiency to cause congenital chronic hemolytic anemia is PK deficiency.45 Its pathophysiology is unclear. PK deficiency affects people worldwide, with a slight preponderance among those of northern European or Chinese descent. Clinical manifestations of the disease vary widely, from transfusion-dependent severe anemia in early childhood to well-compensated mild anemia in adolescents or adults. Diagnosis is made either by a screening test or by detection of specific mutations at the complementary DNA or genomic level. Splenomegaly is common, and in severe cases, splenectomy can alleviate transfusion requirements.5 As with other disorders that cause hemolytic anemia in children, splenectomy should be delayed if possible to at least 4 years of age to reduce the risk of postsplenectomy infection.
The most common red blood cell enzyme deficiency overall is G6PD deficiency. It is far more prevalent than PK deficiency with more than 400 million people affected worldwide, although most experience only moderate health risks and no longevity reduction.46 Clinical manifestations—chronic hemolytic anemia, acute intermittent hemolytic episodes, or no hemolysis—depend on the variant of G6PD deficiency. The mainstay of therapy is avoidance of drugs known to precipitate hemolysis in patients with G6PD deficiency. Transfusions are given in cases of symptomatic anemia. Conventional wisdom is that splenectomy is not indicated in this disease, and certainly the overwhelming majority of patients with G6PD deficiency will neither require nor benefit from splenectomy. However, one report described a small collection of cases of six symptomatic G6PD deficiency patients who had severe hemolytic anemia and required transfusion, all of whom were identified to share a common mutation at exon 10. All underwent splenectomy. A complete response occurred in four patients (transfusion requirement eliminated), and a partial response occurred in one patient (transfusion requirement reduced); no follow-up data were provided for the remaining patient. This study indicates that for a carefully select group of patients with severe hemolytic anemia attributable to G6PD deficiency, splenectomy may be of benefit, although more data must be collected before such a recommendation can be made.46
Autoimmune hemolytic anemias (AIHAs) are characterized by the destruction of red blood cells, whose erythrocyte life span is diminished by autoantibodies leveled against antigens. AIHA is classified as either primary or secondary, depending on whether an underlying cause, such as a disease or toxin, is identified. AIHA is also divided into “warm” and “cold” categories, based on the temperature at which the autoantibodies exert their effect.47 In cold-agglutinin disease, severe symptoms are uncommon and splenectomy is almost never indicated; therefore, this entity is not discussed further in this section. However, warm-antibody AIHA has clinical consequences with which the surgeon should be familiar.
Warm-antibody AIHA, although occurring primarily in midlife, can affect individuals at all ages. The disorder is more common among women, and fully half of warm-antibody AIHA cases are idiopathic. Clinical presentation may be acute or gradual. Findings include mild jaundice and symptoms and signs of anemia. One-third to one-half of patients present with splenomegaly. Sometimes in such cases the spleen is palpable on physical examination. The diagnosis relies on demonstrating hemolysis as indicated by anemia, reticulocytosis, and/or products of red blood cell destruction, including bilirubin, in the blood, urine, and stool. A positive result on direct Coombs’ test confirms the AIHA diagnosis by distinguishing autoimmune from other forms of hemolytic anemia.
Treatment of AIHA depends on the severity of the disease and whether it is primary or secondary. Severe symptomatic anemia demands prompt attention, often requiring red blood cell transfusion. The mainstay treatment for both primary and secondary forms of symptomatic, unstable AIHA is corticosteroids. Therapy should continue until a response is noted by a rise in hematocrit and fall in reticulocyte count, which generally occurs within 3 weeks.
Clinical response to splenectomy for AIHA varies, and the evidence from a number of small case series is conflicting. For example, one 2004 series reported a favorable response to splenectomy in patients with AIHA secondary to chronic lymphocytic leukemia,48 whereas more recent series found no benefit from splenectomy in patients with AIHA secondary to systemic lupus erythematosus or inflammatory bowel disease.49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree