Smooth Muscle Tumors
INTRODUCTION
Tumors with smooth muscle differentiation occur in skin, subcutis, and deep soft tissue as well as pelvis, retroperitoneum (including paratesticular structures), male and female genital tracts, and other viscera. Many of these form distinct clinicopathologic subsets since behavior and criteria of malignancy relate primarily to location. However, the constituent cells have common morphologic and immunohistochemical properties. Some tumors termed myofibroblastoma have characteristics more suggestive of smooth muscle differentiation, but for reasons of terminology and differential diagnosis, they are discussed in Chapter 7.
GENERAL FEATURES OF SMOOTH MUSCLE TUMORS
Pathologic Features
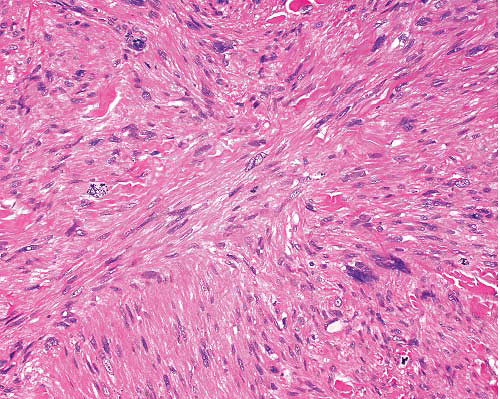
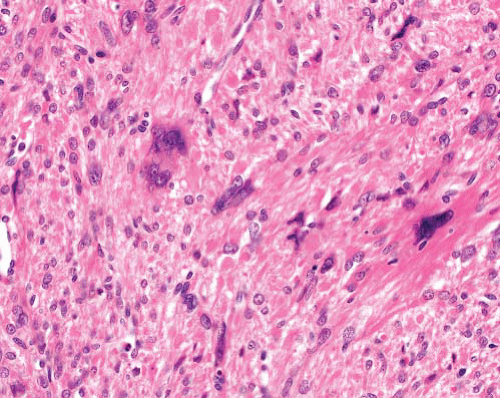
Microscopically, smooth muscle tumors are characterized by elongated cells that are parallel sided with eosinophilic, finely fibrillary cytoplasm, and nuclei which are blunt ended and sometimes have paranuclear vacuoles. The latter are more prominent in sections where the spindle cells are cut transversely. The cells are typically arranged in fascicles disposed at right angles to each other, and nuclear palisading can be seen, imparting a resemblance to schwannoma. Stromal features include separation of the cells by myxoid change or fibrosis (the latter sometimes with calcification) and presence of osteoclast-like giant cells. “Degenerative” nuclear change, characterized by enlargement, irregular shape, and uniform hyperchromasia, is a relatively common feature of smooth muscle tumors. This can be seen without mitotic activity (symplastic leiomyoma) or with a prominence disproportionate to the low level of mitotic activity in low-grade leiomyosarcomas. In higher grades of leiomyosarcoma, there is more diffuse nuclear pleomorphism including prominent nucleoli and more frequent and abnormal mitoses as well as necrosis.
Ancillary Investigations
Smooth muscle tumors are usually diffusely positive for smooth muscle and muscle-specific actins and calponin and at least focally so for desmin
and h-caldesmon. Some smooth muscle tumors additionally express epithelial markers (cytokeratins—often in a dot-like pattern—and epithelial membrane antigen) and S100 protein, but usually in such cases, there is coexistent immunoreactivity for smooth muscle antigens. Occasional leiomyosarcomas, including intra-abdominal tumors, are also CD34-positive but lack CD117 and DOG1, facilitating the distinction from gastrointestinal stromal tumors. Many smooth muscle tumors, especially in females, display positivity for estrogen (ER) and progesterone (PgR) receptors. A subset in the female genital tract, including those with epithelioid morphology, expresses HMB45.1 There are no consistent molecular genetic abnormalities that are diagnostically useful.
and h-caldesmon. Some smooth muscle tumors additionally express epithelial markers (cytokeratins—often in a dot-like pattern—and epithelial membrane antigen) and S100 protein, but usually in such cases, there is coexistent immunoreactivity for smooth muscle antigens. Occasional leiomyosarcomas, including intra-abdominal tumors, are also CD34-positive but lack CD117 and DOG1, facilitating the distinction from gastrointestinal stromal tumors. Many smooth muscle tumors, especially in females, display positivity for estrogen (ER) and progesterone (PgR) receptors. A subset in the female genital tract, including those with epithelioid morphology, expresses HMB45.1 There are no consistent molecular genetic abnormalities that are diagnostically useful.
SMOOTH MUSCLE TUMORS OF SKIN AND SUPERFICIAL SUBCUTIS
Clinical Features
Cutaneous leiomyoma can be solitary or multiple (leiomyomatosis), and a familial form is associated with an autosomal dominant inheritance of mutations in the MCUL-1 gene at 1q42.3-q43, a tumor suppressor gene encoding fumarate hydratase. This genetic abnormality is also implicated in the syndrome of hereditary leiomyomatosis and renal cell cancer.2 The cutaneous lesions occur mostly in young adults on extensor surfaces of limbs as well as trunk and face. They form small pink to brown papules or nodules in a dermatome distribution and can be painful or tender, especially in the cold.
Subcutaneous leiomyoma is rare (see below). Vascular leiomyoma (angioleiomyoma, angiomyoma) is a painful, tender, and cold-sensitive tumor in subcutaneous tissue of head and neck or extremities favoring middle-aged females. A similar lesion has recently been described in the myometrium as a leiomyoma variant.3
Cutaneous and superficial subcutaneous leiomyosarcomas also occur on extensor surfaces of limbs and in the head and neck region but more frequently in older adults and predominantly in males. They present as a firm nodule with or without skin discoloration, depending on the tissue plane involved, and can be painful, ulcerated, or bleeding. In one large series of cutaneous leiomyosarcomas, 27% recurred locally and 7% (four cases) metastasized.4 The latter included one case of dermal leiomyosarcoma with minimal extension into subcutis.
Pathologic Features
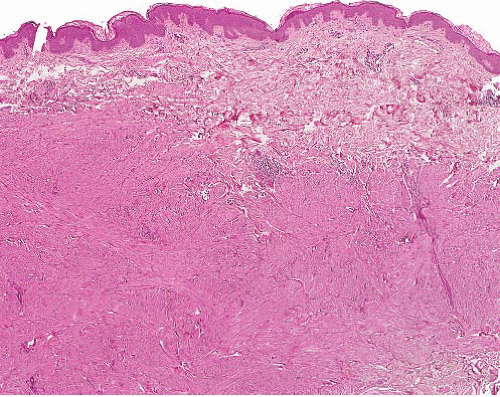
Cutaneous (pilar) leiomyomas are noncircumscribed lesions composed of intersecting fascicles of highly differentiated, closely packed smooth muscle cells that infiltrate dermal collagen (Figs. 6.1 and 6.2, e-Figs. 6.1 and 6.2). The tumor is separated from the epidermis by a grenz zone but sometimes extends into subcutaneous fat.
Vascular leiomyoma is circumscribed, usually <1 cm in diameter, and microscopically is composed of smooth muscle bundles in layers, which blend with muscle walls of blood vessels (Fig. 6.3, e-Figs. 6.3 to 6.7). Three histologic subtypes have been described: solid (with sheets of smooth muscle cells and slit-like vessels), venous (in which smooth
bundles blend with the muscular venous wall), and cavernous (which has minimal extravascular smooth muscle). The morphologic features overlap to some extent with those of myopericytoma, and the two entities are probably related.5
bundles blend with the muscular venous wall), and cavernous (which has minimal extravascular smooth muscle). The morphologic features overlap to some extent with those of myopericytoma, and the two entities are probably related.5
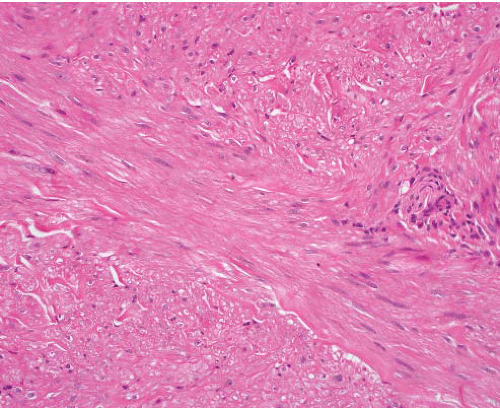 FIGURE 6.2 Cutaneous leiomyoma. The tumor comprises bundles of smooth muscle cells without nuclear atypia, mitotic activity, or necrosis. |
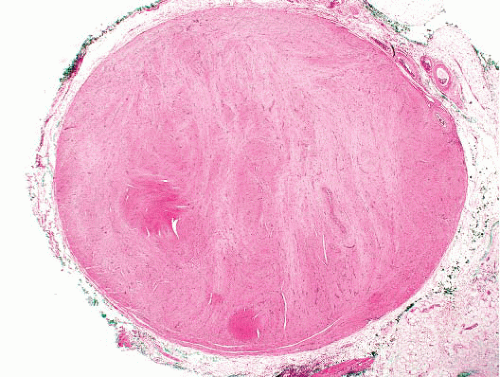 FIGURE 6.3 Angiomyoma. A sharply circumscribed subcutaneous lesion comprising whorls of smooth muscle cells with dispersed slit-like vascular spaces. |
Cutaneous leiomyosarcomas can have a diffuse or nodular growth pattern (Figs. 6.4 and 6.5, e-Figs. 6.8 to 6.11). The former, of presumed arrector pili origin, are usually better differentiated and confined to dermis.
The latter, considered to be tumors of vascular origin, are circumscribed but often more pleomorphic and mitotically active, with deeper extension.
The latter, considered to be tumors of vascular origin, are circumscribed but often more pleomorphic and mitotically active, with deeper extension.
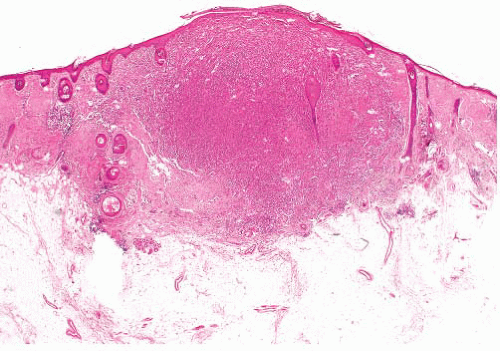 FIGURE 6.4 Cutaneous leiomyosarcoma. The tumor is cellular, infiltrates the dermis, and has irregular margins. Note overlying epidermal hyperplasia with hyperkeratosis and absence of grenz zone. |
Criteria of Malignancy
In cutaneous leiomyomas, the discovery of a single mitotic figure in a section is not conclusive of malignancy, but such lesions should be completely excised with observation in case of recurrence. For cutaneous and superficial subcutaneous lesions, an index of >1 mitosis per 10 hpf, the presence of necrosis, or any significant pleomorphism with or without mitoses indicates a diagnosis of leiomyosarcoma. Knowledge of the precise location of the lesion is essential: leiomyosarcomas involving subcutis have recurrence rates of over 50% and metastatic rates of over 30%, whereas leiomyosarcomas confined to the dermis or very superficial subcutis have high (25% to 50%) recurrent but very low metastatic potential.4 For the latter, the term atypical intradermal smooth muscle tumor has been suggested to reflect this.6 The aim should be to obtain clear excision margins, although there is no precise recommendation for the minimum width of the clearance. Grade is also a prognostic factor, although tumor size and mitotic index alone have less predictive value.
GENITAL LEIOMYOMA
A subset of benign smooth muscle tumors arise in the vulva, scrotum, or nipple. Their features differ slightly according to the site.
Clinical Features
Nipple lesions present in young adults of either sex as a painful nodule involving the areola. Scrotal tumors arise in middle age in the dartos muscle and can form a large painless mass. Vulval leiomyoma occurs in young or middle-aged females as a painless mass, usually in the labium majus, which clinically resembles a Bartholin gland cyst.8
Pathologic Features and Criteria of Malignancy
Leiomyoma of nipple resembles other cutaneous leiomyomas, with infiltrative fascicles of bland smooth muscle cells in mid or upper dermis (Fig. 6.6, e-Figs. 6.12 to 6.14), sometimes with overlying epidermal hyperplasia. Scrotal leiomyomas show similar infiltration of dartos muscle fibers (distinguished from tumor cells by their better organization) but can additionally be hypercellular or show enlarged hyperchromatic nuclei with large nucleoli or multinucleated cells and lymphoid aggregates (Fig. 6.7, e-Figs. 6.15 and 6.16). These bizarre (symplastic) leiomyomas behave in a benign fashion, but the presence of any mitoses in an atypical or hypercellular leiomyoma indicates a diagnosis of leiomyosarcoma. Vulval tumors are often circumscribed rather than infiltrative and can have epithelioid cell morphology and stromal myxoid change or hyalinization. Size >5 cm in diameter, infiltrative margins, moderate to severe cytologic atypia, and a mitotic count of >5 mitoses per 10 hpf are features
indicative of potential malignant behavior in vulval smooth muscle tumors; it has been suggested that the finding of three or more of these features indicates management as leiomyosarcoma, that is, excision with wide negative margins.8 Tumors with one feature are considered benign and those with two are termed atypical leiomyoma; both can be managed by conservative local excision.
indicative of potential malignant behavior in vulval smooth muscle tumors; it has been suggested that the finding of three or more of these features indicates management as leiomyosarcoma, that is, excision with wide negative margins.8 Tumors with one feature are considered benign and those with two are termed atypical leiomyoma; both can be managed by conservative local excision.
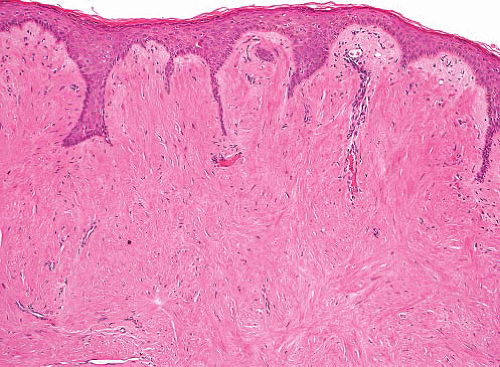 FIGURE 6.6 Leiomyoma of nipple. A homogeneous hypocellular smooth muscle lesion fills the dermis. The cells lack atypia and mitotic activity. |
Ancillary Investigations
In addition to the usual muscle antigens, leiomyomas of both nipple and vulva are frequently positive for ER and PgR. A clonal translocation t(7;8) (p13;q11.2) has been identified in a leiomyoma of vulva.9
SMOOTH MUSCLE TUMORS OF DEEP SUBCUTANEOUS AND SUBFASCIAL SOFT TISSUE
Deeply located smooth muscle tumors present distinct clinical and pathologic challenges and require detailed consideration of the separate entities.
DEEP LEIOMYOMA
Clinical Features
Leiomyomas of deep soft tissue are very rare and should be diagnosed only after careful exclusion of malignant features. They are slowly growing tumors that can reach a large size and form a circumscribed mass. Two types are recognized according to the site: tumors in deep subcutaneous or
subfascial somatic soft tissue and those within the abdomen. Leiomyoma in deep somatic soft tissue arises in limbs, is rarely associated with blood vessel walls, and occurs equally in either sex. Pelvic and retroperitoneal leiomyomas, which resemble uterine leiomyoma, are more common, and the large majority occurs in females.10,11 Like other retroperitoneal tumors, these can grow to a large size before presentation. By definition, in deep soft tissue leiomyoma, there should be infrequent recurrence and no metastases. In two series, no deep somatic leiomyomas and only 3 of 64 (2%) retroperitoneal cases recurred and none metastasized.10,11
subfascial somatic soft tissue and those within the abdomen. Leiomyoma in deep somatic soft tissue arises in limbs, is rarely associated with blood vessel walls, and occurs equally in either sex. Pelvic and retroperitoneal leiomyomas, which resemble uterine leiomyoma, are more common, and the large majority occurs in females.10,11 Like other retroperitoneal tumors, these can grow to a large size before presentation. By definition, in deep soft tissue leiomyoma, there should be infrequent recurrence and no metastases. In two series, no deep somatic leiomyomas and only 3 of 64 (2%) retroperitoneal cases recurred and none metastasized.10,11
Pathologic Features
Microscopically, deep leiomyomas display the usual fascicles of well-differentiated smooth muscle cells and are considered to resemble vascular smooth muscle (Fig. 6.8, e-Figs. 6.17 to 6.25). In somatic deep leiomyoma, there can be clear cell areas, and calcification is common. In retroperitoneal tumors, a range of changes similar to those of uterine leiomyoma can be found. These include hyalinization of blood vessels, focal calcification or ossification, old hemorrhage, and cyst formation. Degenerative nuclear atypia without mitoses (symplastic change) does not indicate malignancy.12,13 In males, retroperitoneal leiomyomas are more cellular with solid and trabecular patterns and occasional clear cell areas, but hyalinization, calcification, and ossification can also be seen.11 Some smooth muscle tumors have a component of differentiated adipose tissue, without atypia. These can be considered as neoplasms with divergent differentiation and are known as lipoleiomyoma or myolipoma according to




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree