Secondary Infrainguinal Arterial Reconstruction
Michael Belkin
David K.W. Chew
Introduction
Infrainguinal arterial reconstruction using autogenous vein is an effective operation for limb salvage in patients with critical limb ischemia. However, despite reported cumulative graft patency rates of 76% at 10 years after infrainguinal bypass surgery, about 20% to 50% of patients will ultimately suffer graft failure. The majority of these patients present with a recrudescence of their original symptoms and in most cases will suffer limb-threatening ischemia. Approximately 10% of our patients undergo major amputation as their next intervention after failure of their infrainguinal bypass grafts. In most patients, however, an attempt at limb salvage through restoration of graft patency or repeat bypass surgery is appropriate.
The challenges of secondary infrainguinal bypass surgery after a previously failed reconstruction are well known to vascular surgeons. Extensive scarring in the operative field and a lack of ipsilateral greater saphenous vein (GSV) necessitating the use of alternative vein conduits present technical challenges to the surgeon. Furthermore, included in this cohort of patients with failed previous reconstructions are those patients with the most severe atherosclerosis, marked intimal hyperplastic response, poor quality venous conduits and hypercoagulable states. Therefore, it is not surprising that the published results of secondary bypass surgery in large series of patients are often inferior to those achieved with primary reconstructions. Given the complexities of secondary bypass surgery, a premium must be placed on effective graft surveillance and when necessary, revision of the vein grafts to maintain bypass graft patency. In this chapter, we will review our graft surveillance protocol and strategies for revision of failing vein grafts. In addition, the selection of patients, operative considerations, and the technical strategies that may contribute to successful secondary arterial reconstructions will also be discussed.
Etiology of Graft Failure
A detailed understanding of the mechanisms of graft failure is important for the management of patients with occluded vein grafts. The etiology of vein graft occlusion generally varies with the interval from operation to the time of graft failure. Early graft occlusions, which occur within the first 30 days after bypass surgery, are usually attributable to errors of operative technique. These include technical errors (e.g., inadequate arterial flushing, clamp injuries, retained valves, vein graft injuries, anastomotic errors, etc.), as well as judgmental errors (employing inadequate inflow or outflow vessels, the use of inadequate venous conduit, etc.). Rarely, early graft thrombosis may be due to graft surface thrombogenicity or unsuspected hypercoagulable states. Vein graft failure from 31 days to 18 months after surgery most frequently results from the formation of intimal hyperplastic lesions within the graft or at the site of an anastomosis. Finally, vein graft failure beyond 18 months after surgery is usually due to the progression of atherosclerotic disease within the inflow or outflow vessels, ultimately leading to graft thrombosis.
Graft Surveillance and Revision
Routine periodic examination of the vein graft using duplex ultrasonography may detect subclinical lesions that predispose to graft thrombosis, permitting prophylactic revision of the graft to prolong its patency. Patient evaluation and duplex examination of the graft are performed at 1, 3, 6, 9, 12 months postoperation and yearly, thereafter. A recurrence of symptoms, change in character of the graft or distal pulses, or a decrease in the ankle-brachial index >0.1 or pulse volume recording waveforms are indications of possible graft stenosis. Duplex ultrasound criteria of impending graft failure include: decreased overall graft velocity (peak systolic velocity (PSV) <25 cm/s in a normal caliber graft), focal increase in velocity (PSV > 300 cm/s), or an increase in PSV in one segment of the bypass greater than three times that of an adjacent segment. A contrast angiogram or magnetic resonance angiogram should be performed to confirm the diagnosis and define the anatomy of the vein graft. Focal lesions (<3 cm) are best addressed using vein patch angioplasty. Occasionally, very short (<15 mm) lesions with flanking segments of normal caliber vein may be percutaneously balloon angioplastied. Long segmental lesions (>3 cm), generally, require interposition vein grafting or jump-grafting around the anastomoses using autogenous vein. Transposition of the vein graft to a different vessel target may also be utilized if such an option exists. A review of our experience with revision of vein bypass grafts showed an overall 5-year primary patency from the time of graft revision of 49% ± 5%, secondary patency of 80% ± 4%, and limb salvage rate of 83% ± 4%. These durable results were similar among the different techniques of graft revision utilized. Tibial/pedal bypass grafts and grafts that were revised within 6 months of the index operation were associated with poorer long-term patency following graft revision.
Management of Early Graft Failure
As noted above, early postoperative vein graft failure is usually due to a technical or judgmental error and results in a recurrence of ischemic symptoms (often more severe than the preoperative state). If the patient is stable and an acceptable risk for reintervention, an attempt at restoration of graft patency is warranted. The etiology of graft failure can often be suspected based on the intraoperative findings at the time of the original operation, as well as a review of the completion arteriogram. For example, the use of a marginal venous conduit or a history of vein wall trauma occurring during valve lysis at the original operation may suggest an etiology for graft occlusion.
When vein graft failure is diagnosed within the first several days after operation, immediate return to the operating room is indicated to minimize adherence of the thrombus to the graft wall, propagation of thrombus into the outflow vessels and ischemic injury to the vein graft itself. The patient is systemically anticoagulated with heparin, the proximal and distal graft hoods are opened and the thrombus is gently extracted using a combination of heparinized saline irrigation through the graft and balloon catheter thrombectomy, when necessary. Limited local instillation of thrombolytic agents within the graft conduit or outflow vessels may be a useful adjunct. All potential defects are corrected with patch angioplasties, interposition grafts or replacement of larger segments of graft with newly harvested vein. Intraoperative duplex scanning is useful to evaluate the graft for any residual defects. Antiplatelet therapy is maintained during and after surgery. Short-term anticoagulation (2 to 4 weeks) is used in all patients, while long-term anticoagulation with warfarin is employed selectively. This is particularly important for the small number of patients in whom no technical defects are identified. These patients have an increased incidence of unsuspected hypercoagulable states, among which anti-phospholipid syndrome and heparin-induced platelet activation correlate most highly with early graft occlusion.
Management of Intermediate and Late Graft Failure
A variety of important factors must be considered when confronted with a patient with a failed infrainguinal reconstruction beyond the perioperative period. If the patient does not have significant rest pain or ischemic ulceration, conservative nonoperative management may be preferred. At the other end of the spectrum are patients whose medical comorbidities and general debility prohibit a major secondary infrainguinal reconstruction. In most patients, however, the goals of relieving pain and preserving function of a critically ischemic limb through a secondary bypass operation are appropriate. The surgeon must decide whether to attempt to restore patency to the failed graft or proceed to a new secondary bypass graft.
Restoration of patency of the bypass graft followed by revision of the graft is often desirable. This is achieved by removal of the thrombus, evaluation of the graft with angiography or duplex ultrasonography, and finally repair of the defects responsible for graft failure. The best results with this approach are achieved when the intervention occurs early (within 30 days) after graft thrombosis in bypass grafts, which have been patent for more than 1 year. Unfortunately, balloon-catheter thrombectomy of thrombosed vein grafts has seldom been rewarded with satisfactory long-term graft patency rates with only 19% to 28% patency rates at the 5-year interval after reintervention for the failed graft.6 The failure of vein graft thrombectomy has led to considerable enthusiasm for the use of thrombolytic therapy in the initial management of patients with thrombosed vein grafts. Thrombolytic therapy offers several potential advantages. Completion of arteriography after successful thrombolysis supplies the surgeon with a “road map” of the vein graft and may reveal the defect(s) responsible for occlusion. Additional advantages include the avoidance of balloon catheter-induced endothelial injury and the potential for more complete removal of thrombus from both the graft and outflow vessels. Following dissolution of the clot, revision of the graft is performed using one of the techniques
listed above depending on the anatomy of the culprit lesion.
listed above depending on the anatomy of the culprit lesion.
In vein grafts that have been thrombosed for more than 30 days, thrombolytic therapy is less successful in restoring normal graft patency. In these cases, especially if the patient experiences a recurrence of critical limb ischemia, a new secondary bypass procedure should be considered.
Surgical Exposure of Inflow and Outflow Vessels
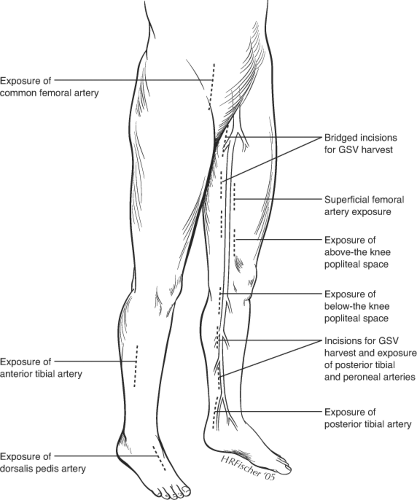
Exposure of previously operated blood vessels poses one of the greatest challenges of reoperative bypass surgery. Dense adherence of the vessels to surrounding scar makes the dissection tedious and time-consuming. As a general rule, minimal dissection should be performed to expose only a sufficient portion of the vessel to control flow and create an anastomosis. Great care must be taken to avoid breaching the outer layers of the arterial wall (sub-adventitial plane), which may compromise the integrity of the vessel wall and require an extensive local repair, such as with a patch angioplasty, before the vessel can be used as inflow or outflow for the new bypass. Scarring is usually most severe at areas of previous anastomotic sites. Prior prosthetic grafts are usually incorporated by a dense fibrotic capsule. It is best to enter this capsule directly with sharp dissection, which then allows easy separation of the graft from the remaining capsule with gentle blunt dissection. Following the graft toward the anastomosis, it is critical to avoid cutting the previous suture line during the dissection as the integrity of the anastomosis will be violated and a pseudoaneurysm may result. Reexposure of native blood vessels is best done by starting in an un-violated plane slightly more proximally or distally, and then following these vessels inward, using careful sharp dissection through scar tissue. Staying as close to the vessel wall as possible without entering the adventitia will avoid potential injury to surrounding structures, such as nerves and veins.
The use of a sterile surgical tourniquet to achieve a bloodless field has greatly simplified the control of previously dissected vessels. This technique is employed for the reexposure of the below knee popliteal artery or more distal vessels. With this technique, only the anterior surface of the vessel, sufficient for completing the anastomosis, needs to be exposed. Circumferential control and dissection of extra length of vessels for placement of clamps or vessel loops are unnecessary. The patient is anticoagulated, the leg is elevated and exsanguinated with an Esmark bandage, and a sterile thigh tourniquet is applied at 250 to 300 mm Hg over a cotton thigh wrap. The outflow vessel is then incised and the distal anastomosis constructed in a minimally exposed surgical field, which is unfettered with clamps or vessel loops.
Given the inherent challenges of reexposure of blood vessels, it is best to avoid redissection of arteries whenever possible, by selecting alternative inflow and outflow sites. Alternative inflow sites may include the superficial femoral artery (SFA) below the femoral bifurcation or the popliteal artery. Even in the setting of prior femoral to distal artery bypasses, the SFA may be relatively well preserved down to Hunter’s canal. This vessel is easily exposed through a medial, mid-thigh incision, which is then carried down through the superficial fascia. The sartorius muscle is retracted posteriorly. The SFA pulse may then be felt through the adductor fascia, which is then incised (Fig. 1). The profunda femoris artery (PFA) beyond the lateral circumflex artery may also be suitable as an inflow vessel and can be exposed by incising lateral to the sartorius muscle and carrying the dissection down between the superficial femoral vessels and the adductor longus muscle.
In selected situations, the proximal common femoral or distal external iliac artery may be used as the inflow artery. Although these sites have the disadvantage of
lengthening the venous conduit necessary for bypass, they are easily exposed and are particularly suitable when the distal anastomosis may be constructed at the popliteal level. These more proximal inflow vessels should only be employed when there is unimpeded inflow into the PFA. Since long-term limb salvage often hinges on patency of the PFA, this vessel should be reexposed and reconstructed (with a profundaplasty), whenever necessary as part of the secondary bypass procedure.
lengthening the venous conduit necessary for bypass, they are easily exposed and are particularly suitable when the distal anastomosis may be constructed at the popliteal level. These more proximal inflow vessels should only be employed when there is unimpeded inflow into the PFA. Since long-term limb salvage often hinges on patency of the PFA, this vessel should be reexposed and reconstructed (with a profundaplasty), whenever necessary as part of the secondary bypass procedure.
Avoidance of redissection is particularly important in exposure of distal outflow vessels. Reexposure of tibial vessels is easily complicated by trauma to the arteries and accompanying venae comitantes. The tibial veins are often densely adherent to the artery and resultant bleeding can make the exposure extremely difficult. In the majority of cases, it is possible to expose new, more distal (or even more proximal) sites on the vessel. Similarly, alternative routes of exposure (such as lateral exposure of the peroneal artery with segmental fibular resection) may simplify exposure of distal vessel targets.
Operative Strategies
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree