Regulation of Growth of Gastric Epithelium
Introduction
Disease occurs because of disruption or malfunction of the different barriers of the upper gastrointestinal tract. Recovery from damage depends on the properties of the epithelial cells and their ability to repair injury.
The gastric mucosa contains several cell types, each programmed to undergo cell division at a specific rate and to equal this rate of cell division by an equal rate of apoptosis. Increased apoptosis without change in cell division results in atrophy; increased cell division without change in apoptosis results in hyperplasia. It is an exquisitely modeled epithelium with distinct regions containing specific cell types. Clearly, maintenance of differentiation to generate the normal epithelium is quite specific and requires a precise programming of growth, differentiation, and apoptotic factors.
The cells of the gastric fundic epithelium that appear clinically relevant are the surface epithelial cells, the neck cells, the parietal and chief cells, and the endocrine cells, mainly the ECL and D cells. In the antrum, there are the surface cells, the neck and gland cells, the D and G cells, and a chief cell that secretes a different isoform of pepsinogen. The neck region of the glands contains the progenitor cells for the surface, parietal, and peptic cells.
Rather little attention was paid to growth regulation of many of these cell types until the development of modern knowledge of gastrin. Gastrin was originally considered only of relevance in terms of stimulation of acid secretion, but Rusty Johnson showed in the early 1970s that gastrin was trophic for the fundic, but not the antral, epithelium. Since then, much effort has been expended into a better definition of the mechanism of action of gastrin, at first on the ECL cell and later on the parietal/peptic cells. Several other trophic factors have now been shown to be present or induced after epithelial damage, but it is far too early to even begin to define the program that allows expression of the mature gastric epithelium with its multiple cell types in the right place at the right time.
Cell growth in the stomach
The rat stomach has formed the primary in vivo model for studies of the growth regulation of the epithelium, usually by measurement of DNA synthesis (3H thymidine or BrdU incorporation).
Starvation for 24 or 48 hours results first in a degree of fundic atrophy. Refeeding then produces a large increase in DNA synthesis in the neck cells of the fundic glands, which contain the progenitor cells for the surface and the glandular structures. There is also incorporation of these markers into ECL cells scattered throughout the fundus. Refeeding is several-fold more effective than gastrin, showing that gastrin is overshadowed by other factors in this particular model.
The transit time of surface epithelial cells is approximately 72 hours, whereas that of parietal and chief cells is approximately 90 to 120 days.
There is detailed knowledge of cell lineage and growth in the intestinal mucosa not matched by knowledge of these properties of the gastric mucosa.
There are stem cells, very few in number, that divide slowly to provide daughter cells. One of these two cells is destined to remain a stem cell; the other divides to form preprogenitor cells that, in turn, divide to form progenitor cells, which then form cell-type precursors and finally the mature cell type. This program is held within the cells and outside the cells by external factors generated by other cell types. The stem cells of the gastric mucosa are pluripotent.
The generation of parietal cells is in the neck region, and it is possible to recognize preparietal or progenitor cells, which then differentiate to form a triple helix chain of cells down the length of the gastric gland. Parietal cells are recognizable by their large mitochondrial content (34% cell volume) as well as by expression of the gastric acid pump and of the histamine-2 and CCK2 receptors. They are end cells and have not been shown capable of cell division.
Chief cells are found generally deeper in the fundic gland, mostly in the bottom third. They are recognizable because of their high content of pepsinogen and expression of the CCK1 receptor subtype. They appear to have some residual capacity for division. There is currently no explanation for their anatomic localization. A slower generation time than that of the parietal cells would enable deeper localization of these cells.
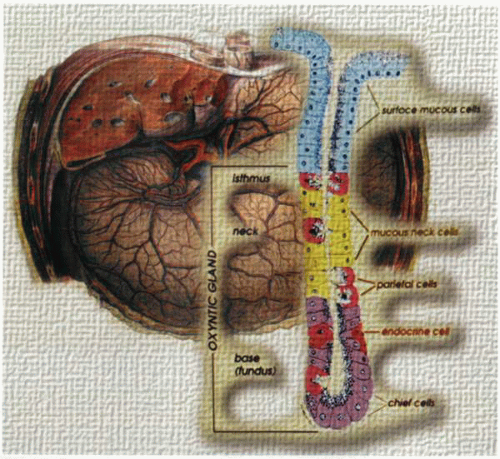 A cartoon of the fundic gastric gland (lower right) demonstrating the location and relationship of the different cell types within the stomach (background). |
ECL cells are the major endocrine cells of the fundic mucosa and are found toward the lower third of the gastric fundic gland. In animals, these cells are clearly capable of cell division. Some claim that the ECL cell in the human mucosa is also an end cell like the parietal cell, but it is unusual to find a wide gap between humans and other mammals in something as fundamental as capacity for cell division.
In recent years, the ECL cell has achieved prominence as the target for gastrin’s hyperplastic effect on the endocrine cell population in the stomach. ECL cells are easily identified due to their histamine, acidic vacuole, and HDC content.
Growth receptors in gastric mucosa
A large number of factors have been shown to stimulate gastric cell replication in vivo and in gastric cell lines in vitro. Surely, a well-conducted orchestra of these factors is necessary for the generation and regeneration of a normal gastric epithelium. How the integration of all these factors enables replacement of the mature epithelium remains a challenge for the future, as it does in all organ biology.
Gastrin
This is the best studied of the various factors influencing growth in the fundus of the stomach. Recent investigations using CCK2 receptor KNO mice have confirmed the critical role of gastrin/CCK in mucosal growth. Although capable of stimulating ECL cell replication, it is less effective than many other factors in driving gastric cell replication. On the other hand, the hypergastrinemia induced by treatment with PPIs can result, in animals and in some patients, in parietal and chief cell hyperplasia as well as ECL cell hyperplasia.
It has been repeatedly demonstrated, however, that these are without clinical consequence in the 15 years that maintenance omeprazole studies have been present. An even longer period of surveillance of selective or highly selective vagotomy patients, in whom hypergastrinemia results with approximately the same elevation found in maintenance PPI therapy, has shown no unexpected changes in gastric mucosal cells. The apparent affinity for the growth receptor on the ECL cell appears to be approximately 50-fold higher than the affinity for the receptor activating histamine release from the same cell type. Neutralizing antibodies to gastrin result in at least 50% inhibition of the feeding response on gastric DNA synthesis, arguing for a continuing important role of this hormone in gastric growth regulation.
Why there is not a trophic action of gastrin on the antrum is completely unknown. As discussed elsewhere, gastrin also regulates HDC expression in ECL cells.
Epidermal growth factor and transforming growth factor-α
EGF and TGF-α are peptide hormones that are potent stimuli for cell division in the gastric mucosa and also potent inhibitors of gastric acid secretion. The former action is undoubtedly due to a tyrosine kinase action, and the latter is due to extracellular signal-regulated kinases (ERKs) 1 and 2 activation.
The inhibition of acid secretion that occurs at the same time as stimulation of growth suggests that the presence of acid secretion is inimical to growth. TGF-α is upregulated in the vicinity of gastric ulcers, suggesting a real physiologic role for this peptide and its receptor in ulcer healing.
The inhibition of acid secretion that occurs at the same time as stimulation of growth suggests that the presence of acid secretion is inimical to growth. TGF-α is upregulated in the vicinity of gastric ulcers, suggesting a real physiologic role for this peptide and its receptor in ulcer healing.
Hepatocyte growth factor
Hepatocyte growth factor (HGF) is also a potent mitogen, acting at a c-met receptor. It stimulates expression of the vascular endothelial growth factor (VEGF) receptor for endothelial cell growth and permeability. It is implicated as one of the mediators in mucosal repair.
Keratinocyte growth factor
Keratinocyte growth factor (KGF) is a peptide that also significantly stimulates division of gastric cells in vivo and in vitro, although its site of production in the gastric mucosal vicinity has not been established.
Transforming growth factor-β1, -2, and -3
TGF-β1, -2, and -3, in general, inhibit cell division in the gastric mucosa and show differential regulation among the different cell types. TGF-β1 is localized exclusively to the parietal cells, β2 is found in chief cells, and β3 in parietal, chief, and mucous cells. Their role in ulcerogenesis is unclear.
Cytokines
Increased expression of a variety of interleukins has been found in gastric mucosa subsequent to infection by H. pylori, such as interleukin (IL)-1β and IL-8. The former has clearly been shown to inhibit cell growth in the stomach. Which of these plays a role in the ulcerating damage caused by the organism is not entirely clear.
Ammonia (NH3)
H. pylori produces large quantities of NH3 from urea, especially during periods of acid secretion. In vitro, NH3 has been shown to inhibit cell growth, induce alkalinization of the cell interior, and increase intracellular Ca2+. This product of infection may exert pleiotropic negative effects on the gastric mucosa. The action of NH3 entry into cells is alkalinization of the cell interior. This leads to unloading of calcium stores that are IP3-sensitive but also leads to partial unloading of other stores, such as those in mitochondria. This latter event may be proapoptotic.
Epithelial restitution
The term epithelial restitution describes the finding that surface epithelial cells migrate over the surface of a gastric wound without undergoing cell division. HGF is a stimulant of restitution, which is the result of the changed expression of a variety of integrins. The targeting scaffold on which restitution rests is an important but as-yet unexplored area of gastric biology.
Mucosal healing and trefoil peptides
The mucosal lining of the stomach, and the epithelial cells in particular, undergoes constant renewal. Thus, new cells are created from precursors in the proliferative zone and under the influence of “growth factors” undergo maturation and differentiation over the course of a 5- to 7-day period while they move toward the luminal surface. At this site, they perish, not necessarily by passive shedding into the lumen, but by an active process of cell death termed apoptosis. Indeed, this may be considered the ultimate terminal-differentiation event. In the gastric mucosa, cells also move downward (at a much slower rate) from the proliferative zone at the base of the gastric crypts into the glands. This constant and rapid turnover of the stomach mucosa is well suited to the prompt restoration of mucosal integrity after damage that may occur during acid peptic disease.
After the establishment of a mucosal breach or ulcer, three main healing events have been defined. The first is a rapid phase of epithelial restitution during which existing viable epithelial cells at the ulcer edge migrate inward to close the gap. Second, over the next few days, new cells are formed by proliferation to repopulate the mucosal gap. Third, new matrix is laid down as nonepithelial cells in the lamina propria replace inflammatory cells. This remodeling is accompanied by angiogenesis. The migrating epithelial cells are not merely undifferentiated and immature but instead have a specialized and polarized phenotype uniquely adapted to movement. The precise differentiation of this migratory phenotype is influenced by a number of factors, including at least the extracellular matrix, peptides, and the more classic growth factors. It has been established that a chronic and repetitive mucosal insult, as opposed to an acute event, significantly influences epithelial restitution. Thus, an acceleration of restitution may occur as an adaptive response, as compared to that found in “unconditioned” mucosa, and is associated with a widening of the proliferative zone.
This process also appears to involve a decrease in parietal and gastrin-secreting cells and a tendency to increase somatostatin-secreting cell numbers. A further change is the generation of a new population of “vesiculated” cells, some of which have features of immature mucous neck cells. These are present in the
lower third of the gastric gland in an area previously populated by parietal cells. Such vesiculated cells may represent a distinct lineage of cells ideally located to promote proliferation and healing through the local secretion of mucosal repair proteins. The relationship between this observation and the reports of an ulcer-associated cell lineage (UACL) by Wright and his colleagues may be an important issue in the elucidation of mucosal repair in the upper gastrointestinal tract.
lower third of the gastric gland in an area previously populated by parietal cells. Such vesiculated cells may represent a distinct lineage of cells ideally located to promote proliferation and healing through the local secretion of mucosal repair proteins. The relationship between this observation and the reports of an ulcer-associated cell lineage (UACL) by Wright and his colleagues may be an important issue in the elucidation of mucosal repair in the upper gastrointestinal tract.
Of considerable importance is the identification of the molecules, which are critical to the regulation of mucosal repair. In more recent times, a number of key mucosal-repair peptides have been identified and classified according to their function. EGF is capable of performing many of the functions essential for good repair and is considered an example of a luminal-surveillance peptide. Although secreted into both serosal and luminal sides, it is only able to bind to its receptor located on the basolateral surface of the epithelial cell when mucosal integrity is breached. On the other hand, pancreatic secretory trypsin inhibitor and TGF-α are considered mucosal-integrity peptides, ensuring normal barrier function.
The third group, the trefoil factors, appears to act as rapid-response molecules upregulated at times of injury. Of particular relevance to the healing process is a distinct glandular constituent—the UACL—that produces several repair peptides, including trefoil peptides and EGF, as well as mucus. It has therefore been suggested that these cells act as a “first-aid kit,” pouring healing agents onto the ulcer base. The UACL is present only at the site of chronic mucosal injury and probably arises from the duct region of metaplastic epithelium, such as intestinal metaplasia in the stomach.
The trefoil peptides are a highly conserved group of molecules that are widely distributed in the gastrointestinal tissues, although they have been demonstrated elsewhere in sites as disparate as the breast and lung. It is likely that their primary role, however, is in the gastrointestinal tract. The group takes its title from the characteristic trefoil motif, a three-loop structure secured by disulfide bonds based on cysteine residues. The super-secondary structure of the trefoil motif has been examined by three-dimensional nuclear magnetic resonance, demonstrating that it consists of a seven-residue length of alpha helix followed by a short antiparallel β sheet formed from two strands of four amino acids each. This novel structure clearly identifies the trefoil motif as a new class of module distinct
from other types of highly disulfide cross-linked domains, such as those found in EGF and insulin-like growth factor-1 (IGF-1).
from other types of highly disulfide cross-linked domains, such as those found in EGF and insulin-like growth factor-1 (IGF-1).
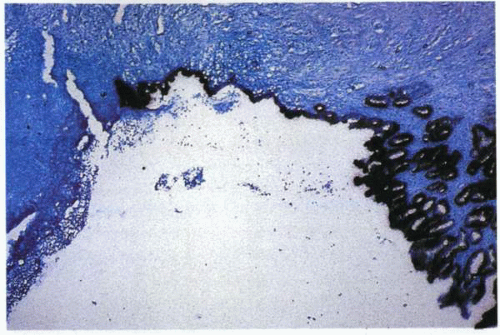
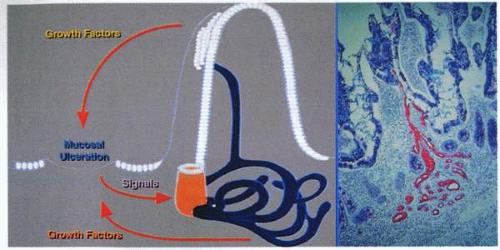 A schematic representation (left) of the development of the “ulcer-associated cell lineage” evident in the photo micrograph (right). |
pS2 was the first molecule characterized and consists of a 60-amino acid peptide with a 24-amino acid signal peptide and is homologous with pancreatic spasmolytic polypeptide (PSP). Although found in abundance in human breast cancers, the predominant pS2 gene expression is most evident in the surface and foveolar cells of the stomach. pS2 is found in normal gastric juice and is secreted by the surface and foveolar cells. Human SP is coexpressed with pS2 in gastric foveolar cells and is also expressed abundantly by the basal antral glands. Both these peptides have been demonstrated to be widely expressed in gastrointestinal tissues during disease states, particularly those related to chronic ulcerative conditions. More recently, studies have demonstrated that what was previously regarded as pyloric metaplasia in chronic gastrointestinal ulceration is, in fact, a differentiating cell lineage.
This buds initially from the bases of intestinal crypts adjacent to the ulcer, and its tubules ramify in the lamina propria before emerging from the mucosal surface through a newly formed duct, which in the small intestine grows upward through the core of an adjacent villus to emerge through a pore in its side. The newly formed gland thus passes its secretion to the surface, and cells from the lineage pass out through the pore to displace the indigenous cell lineage and then close the villus surface. As the cells migrate through the tubular system, they acquire differentiation antigens and also develop a proliferative zone within the duct itself.
The glandular portion of this UACL secretes immunoreactive EGF/urogastrone that is then available to combine with its receptors and to stimulate mucosal healing. It is likely that this process or variations of it represent a basic template whereby mucosal healing of the gastrointestinal tract mucosa occurs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree