Rare Primary Thyroid Epithelial Tumors
Lester D.R. Thompson
MUCOEPIDERMOID CARCINOMA
Mucoepidermoid carcinoma (MEC) is a malignant epithelial neoplasm. By definition, an MEC contains a combination of mucinous and epidermoid components.1 There are two histologic variants of MEC: mucoepidermoid carcinoma and sclerosing mucoepidermoid carcinoma with eosinophilia. The sclerosing variant with eosinophilia will be described in the next section.
Etiology
Histogenesis and Molecular Genetics
The hypothesis that MEC derives from follicular epithelial cells or from the ultimobranchial body remains unconfirmed.4,5,6,7 The detection of squamous metaplasia within papillary thyroid carcinoma and in MEC suggests a follicular derivation. Furthermore, a transition from follicular epithelium is supported by the neoplastic cells reacting with thyroglobulin, thyroid transcription factor 1 (TTF1), TTF2, paired box gene 8 (PAX8), and specific keratin reactions in MEC.8,9 Lymphocytic thyroiditis with squamous metaplasia is present in the background of most cases of MEC, another finding that supports a thyroid follicular epithelial derivation.4 Some authors believe that MEC may in fact be a variant of papillary carcinoma, or at the least very closely related.3,6,10,11,12,13 In contrast to the cells in MEC, solid cell nests (SCNs) typically lack intercellular bridges and are thought to be remnants of the ultimobranchial body, the structure that gives rise to C-cells rather than follicular cells (see Chapter 1). In addition, there is no calcitonin or chromogranin immunoreactivity in MEC. SCNs have mucin-positive debris in their centers and rounded bodies in the apical portions of cells. Finally, MECs may arise in the isthmus and pyramidal lobes, locations in which SCNs are not found.4,7
Clinical Presentation
MEC accounts for <0.5% of all thyroid gland malignancies. Women are affected more frequently than men (2:1). There is a bimodal age distribution at 20 to 40 years and 60 to 80 years. Patients are euthyroid and present clinically with a firm, painless mass in the thyroid gland. Recurrent laryngeal nerve compression is not common.1,4,10,16
Pathology
Gross Presentation
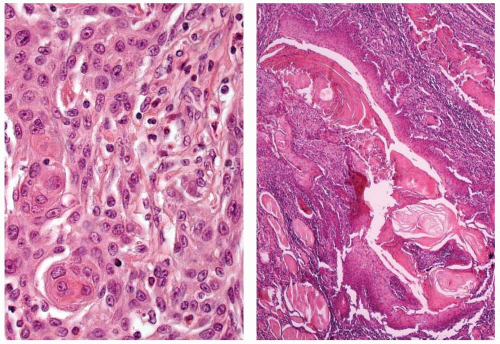
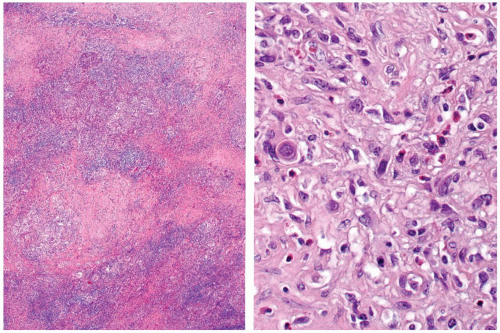
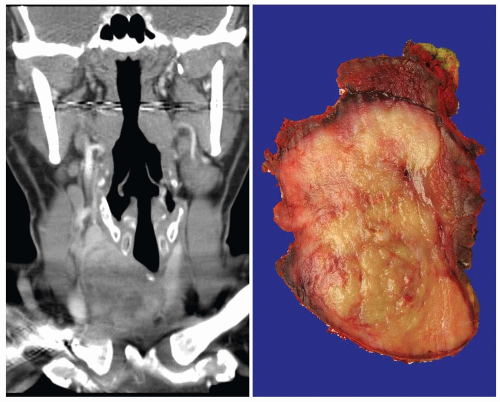
Extrathyroidal extension is noted in up to 20% of patients.4,13,17 The tumors can be as large as 10 cm in their greatest dimension, and they feature a firm cut surface with a tan-brown to yellow-white mass. Although they are well demarcated, they are seldom encapsulated (Fig. 15.1). Cystic degeneration, sometimes with a myxoid-mucoid appearance, and necrosis are sometimes identified.1,4
Microscopic Description
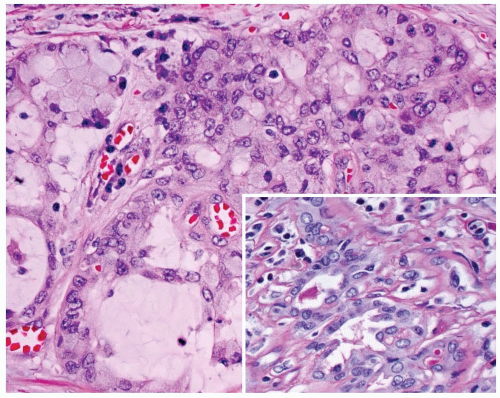
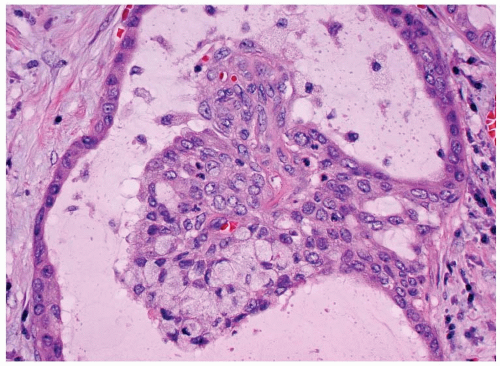
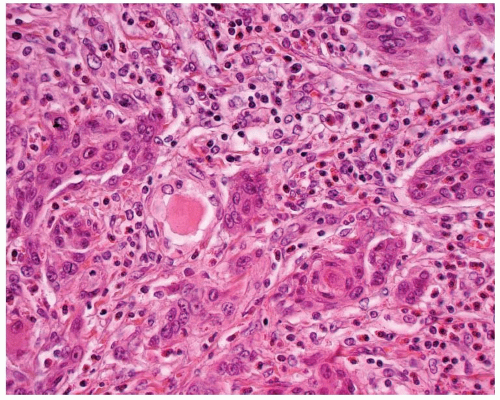
The tumor is infiltrative, demonstrating intertwined cords and nests of epidermoid cells and mucocytes in a stroma of fibrous connective tissue (Fig. 15.2). The epidermoid component may appear as sheets of atypical cells, sometimes with keratinization (Fig. 15.3). Keratin pearl formation is noted. Cystic spaces may be present; when they are, they frequently contain keratinaceous and mucinous debris with inflammatory cells (Fig. 15.3). The goblet-like cells may be seen within the cystic spaces, lining the cords of epidermoid cells, or within gland-like lumina (Fig. 15.4). The cytoplasm of the mucocyte may be clear to foamy or vacuolated. Mucin can be intra- and/or extracellular (Fig. 15.5). Ciliated cells may be seen.1,4 Hyaline bodies (positive with periodic acid-Schiff [PAS] staining) resembling colloid may be seen in the cytoplasm of mucocytes.12 The cells are intermediate in size. Their nuclei have pale nuclear chromatin similar to papillary thyroid carcinoma. Nuclear grooves and intranuclear cytoplasmic inclusions can be seen.1 Psammoma bodies are occasionally present.9,12 Lymphocytic thyroiditis is usually present in the surrounding thyroid parenchyma, and although germinal center formation is common, oncocytic metaplasia of the follicular epithelium is not.1,4,10,12,16 Squamous metaplasia is seen in lymphocytic thyroiditis (Hashimoto thyroiditis) and is present in papillary thyroid carcinoma. Areas of transition between MEC and papillary thyroid carcinoma can be seen.1,6,8,18,19 Concurrent papillary carcinoma has been identified in up to 50% of cases (Fig. 15.6).4,10,12,19 In rare cases, anaplastic (undifferentiated) carcinoma may develop, a finding that often alters the prognosis.10,11,12,20
Immunohistochemistry and Molecular Diagnostics
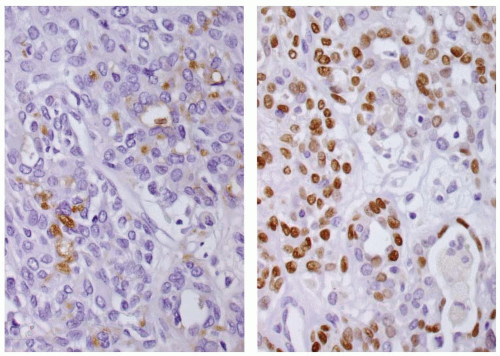
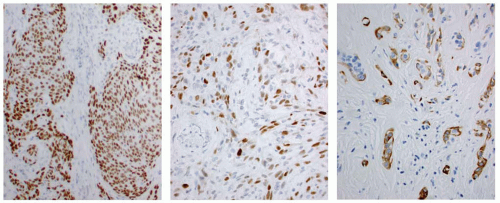
The mucinous material is highlighted with PAS (Fig. 15.4), mucicarmine, and Alcian blue pH 2.5.12 The tumor cells are positive with keratins (high and low molecular weight), with polyclonal carcinoembryonic antigen (CEA) (mucocytes only), and focally with thyroglobulin and TTF1 (Fig. 15.7).1,4,9,16,19,20 p63 and CK5/6 may be of value in highlighting the epidermoid epithelium (Fig. 15.7). P-cadherin neoexpression and E-cadherin abnormalities are seen in the epidermoid tumor cells.21 Calcitonin is negative.4,16,19 Expression of thyroid-specific genes (TTF1, TTF2, PAX8, and thyroid peroxidase) detected by RT-PCR supports a follicular epithelial derivation,9 although BRAF (V600E) mutation is not detected.14,22 The CRTC1/MAML2 fusion is detected in about one-third of cases.15
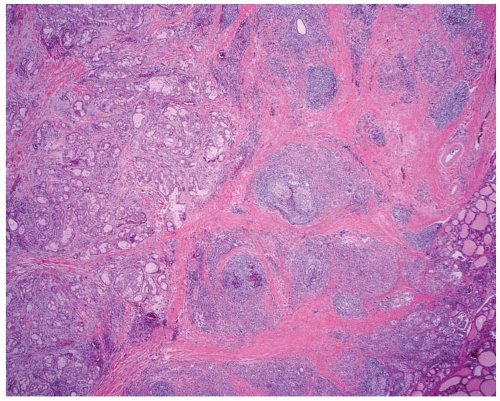 FIGURE 15.1. Mucoepidermoid carcinoma. A low-power magnification demonstrates poor circumscription with heavy fibrosis and a background of lymphocytic thyroiditis. |
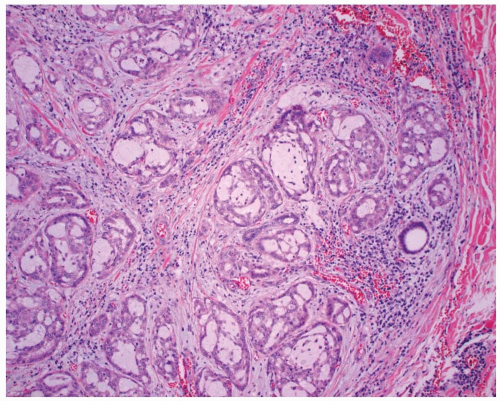 FIGURE 15.2. Mucoepidermoid carcinoma. There are intertwined cords, nests, and glands of neoplastic cells set in a background of lymphocytic thyroiditis. |
Cytopathology
Smears are cellular, with cohesive monolayered and syncytial sheets of cells within a background of amorphous debris and necrotic and mucinous material. A dual cell population of mucocytes (vacuolated to foamy cytoplasm compressing the nucleus) and epidermoid cells (polygonal cells with distinct cell borders and round nuclei, vesicular nuclear chromatin, prominent nucleoli, and opaque, homogeneous eosinophilic cytoplasm) are intermingled.1,3,20,23,24 In rare cases, anaplastic cells may also be present.11,20
Differential Diagnosis
Prominent squamous metaplasia can be seen in lymphocytic thyroiditis, but a tumor mass is not identified. Prominent SCNs may also have mucocytes and mucinous detritus present in the cystic lumina. But SCNs usually appear as isolated microscopic foci rather than as a clinical
mass. Squamous cell carcinoma (SCC) is characterized by marked cytologic atypia, but it lacks mucocytes and extracellular mucin.25 Furthermore, there is usually no associated papillary carcinoma. Squamous metaplasia can be seen in papillary carcinoma, but mucocytes and areas of transitional type epithelium are not seen in papillary carcinoma.
mass. Squamous cell carcinoma (SCC) is characterized by marked cytologic atypia, but it lacks mucocytes and extracellular mucin.25 Furthermore, there is usually no associated papillary carcinoma. Squamous metaplasia can be seen in papillary carcinoma, but mucocytes and areas of transitional type epithelium are not seen in papillary carcinoma.
Treatment and Prognosis
Surgery is the treatment of choice, but if there is extensive local invasion, external beam radiation or radioablation can be employed.8 Many consider MEC of the thyroid gland to be an indolent tumor with a good long-term prognosis (similar to that of papillary thyroid carcinoma).1,4,10 Although death from tumor may occur in older patients, it is usually limited to those cases in which an anaplastic or undifferentiated component has been identified histologically.10,11,12,20,26 Lymph node metastasis, which has been seen in up to 40% of patients,1,4,9,12,13,20 is much more common than distant metastasis, although lung, bone, and pleural metastases have been reported.3,23
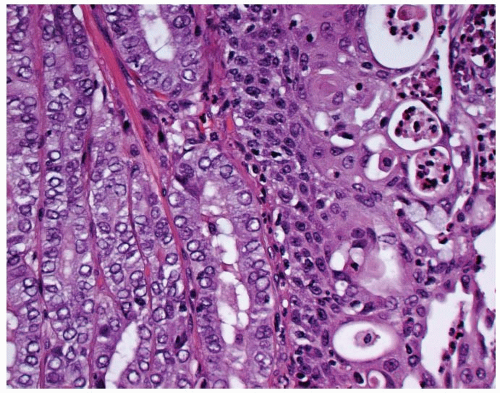 FIGURE 15.6. Mucoepidermoid carcinoma. There is a concurrent papillary carcinoma (left). Notice the mucocytes (right). |
SCLEROSING MUCOEPIDERMOID CARCINOMA WITH EOSINOPHILIA
Sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) is an extremely rare primary thyroid gland neoplasm. It exhibits epidermoid and glandular differentiation set in a prominently sclerotic background with a rich investment of eosinophils and lymphocytes.27,28 It is distinct from MEC, although eosinophils can be seen in both types of tumor.
Etiology
Histogenesis and Molecular Genetics
The tumor is presumed to arise from squamous metaplasia of the thyroid follicular epithelium, usually in the setting of lymphocytic thyroiditis (Hashimoto thyroiditis).27,29 Origin from the ultimobranchial body is a possibility,30 but immunohistochemistry results are equivocal because p63 immunoreactivity is seen in squamous metaplasia and in ultimobranchial body remnants (SCNs).31
Clinical Presentation
Pathology
Gross Presentation
Microscopic Description
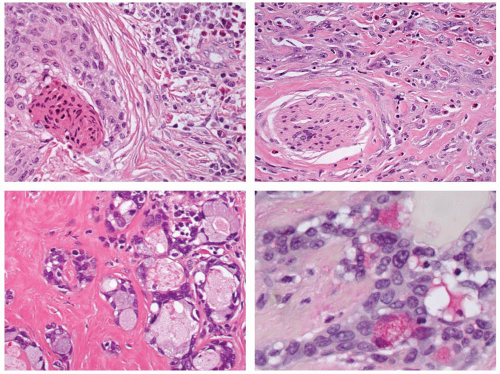
Anastomosing cords, strands, and nests of tumor cells are identified in a stroma of very sclerotic fibrohyaline connective tissue (Fig. 15.8). Innumerable eosinophils are seen throughout (Fig. 15.9). The neoplastic cells are biphasic, with cohesive nests of epidermoid, polygonal cells with well-developed cell borders. Intercellular bridges are noted, with opacified, thick eosinophilic cytoplasm. Keratin pearls and keratin debris can be identified (Figs. 15.9 and 15.10) There is only mild to moderate pleomorphism. Nucleoli are prominent. Occasionally, clear cells are present.29 Mucocytes with squashed nuclei are intermingled in the epidermoid sheets, highlighted with mucicarmine stain or PAS with diastase (Fig. 15.10). Mucus pools are uncommon.27 In rare cases, the tumor cells may grow in a pseudoangiomatous pattern. Extrathyroidal extension is seen in as many as one-half of all cases, with lymph node metastasis in about one-third.10,27,28,36,37 Perineural and vascular invasion is common. Lymphoid infiltration (lymphocytes and plasma cells), including a finding of lymphocytic thyroiditis (Hashimoto thyroiditis), is seen in the adjacent thyroid parenchyma in nearly all cases. Squamous metaplasia is also seen in the areas of lymphocytic thyroiditis.10,27,33,35 Papillary carcinoma is identified in a few cases, although transition between the two is less common than it is in MEC.
Immunohistochemistry and Molecular Diagnostics
Cytokeratins are positive in the tumor cells (Fig. 15.11); TTF1 is identified less often. CEA is sometimes expressed in the mucocytes but not in the areas of squamous differentiation. Calcitonin and thyroglobulin immunoreactive is not present.10,27,28,29 p63 strongly stains the epidermoid tumor cells (Fig. 15.11),30 a finding that is also seen in the basaloid cells of squamous metaplasia.31 TP53 staining is occasionally seen in the epidermoid cells. BRAF mutations are not identified.22
Cytopathology
In general, a definitive diagnosis by fine needle aspiration cytology is difficult because of the nonspecific nature of the findings. There tends to be a combination of malignant epithelial cells set
in a mucinous and debris-filled background with eosinophils. Cohesive clusters of cells with either epidermoid or glandular differentiation are present. These findings suggest malignancy, but they may also raise the possibility of metastatic tumor or Hashimoto thyroiditis.35,38
in a mucinous and debris-filled background with eosinophils. Cohesive clusters of cells with either epidermoid or glandular differentiation are present. These findings suggest malignancy, but they may also raise the possibility of metastatic tumor or Hashimoto thyroiditis.35,38
Differential Diagnosis
Anaplastic (undifferentiated) thyroid carcinoma, SCC, carcinoma showing thymus-like differentiation (CASTLE), Hodgkin lymphoma, direct extension of adjacent organ tumors, and florid squamous metaplasia enter the differential diagnosis.27 Undifferentiated carcinoma has more pleomorphism, sheetlike growth, increased mitotic activity, and necrosis, and it lacks mucocytes and significant eosinophils. Pure SCC has significantly greater pleomorphism, sheetlike growth, and necrosis, and it lacks mucocytes and mucin pools.25 CASTLE can have mucinous material, but it is spindled, tends not to have lymphoid infiltrate, and has a unique immunohistochemical profile. Hodgkin lymphoma may have a rich eosinophilic infiltrate and contains lymphocytes and plasma cells, but it also has Reed-Sternberg cells, which are not present in SMECE.39 Primary carcinomas of the larynx and esophagus can invade the thyroid gland, but in general, the clinical and/or radiographic findings will help make this distinction. Florid squamous metaplasia tends not to form a mass, is present in lymphocytic thyroiditis, and does not have mucocytes or eosinophils.31,40
Treatment and Prognosis
Total thyroidectomy is the treatment of choice, especially since extrathyroidal extension is common. Selected cervical lymph node sampling is recommended for patients with clinically apparent lymph node enlargement—up to 30% of cases at presentation.10,29,34,35,36,37 Lung, liver, and bone metastases are uncommon.35,36,37 The tumor is usually indolent, and the long-term clinical prognosis is intermediate.10,28,33 Specific prognostic factors are unknown.
SQUAMOUS CELL CARCINOMA
A primary SCC in the thyroid gland is composed entirely of cells with squamous differentiation, without mucocytes, and without direct invasion from an adjacent organ (larynx, trachea, or esophagus).41 Therefore, endoscopic evaluation (laryngoscopy, esophagoscopy, bronchoscopy) and radiographic studies are often required to establish a definitive diagnosis.42,43,44,45
Histogenesis and Molecular Genetics
SCC is thought to arise from thyroid follicular epithelium, either directly or through squamous metaplasia, which then undergoes additional alterations to yield a malignant tumor. The squamous epithelium may represent a persistence of thyroglossal duct or branchial pouch remnants. Association with Hashimoto thyroiditis may also be seen.41,44,46,47,48,49 Previous radiation has been reported as an etiologic factor in a few cases.45,50
Clinical Presentation
SCC accounts for <0.5% of all malignant thyroid tumors, with fewer than 100 reported cases.51 Its clinical presentation is similar to that of undifferentiated carcinoma. In both cases, there is a rapidly enlarging neck mass that is very often associated with recurrent laryngeal nerve compression and pressure symptoms, including airway obstruction and dyspnea (Fig. 15.12). Cervical lymph node enlargement is common.52 Most affected patients are in the sixth or seventh decade of life, and the
female-to-male ratio is 2:1.25,43,44,45,48,49,52,53,54,55,56,57,58,59 Hashimoto thyroiditis is concurrently identified in a few patients.25 The paraneoplastic syndrome of hypercalcemia, fever, and leukocytosis is rare and probably develops as a result of tumor-derived humoral mediators.60,61,62
female-to-male ratio is 2:1.25,43,44,45,48,49,52,53,54,55,56,57,58,59 Hashimoto thyroiditis is concurrently identified in a few patients.25 The paraneoplastic syndrome of hypercalcemia, fever, and leukocytosis is rare and probably develops as a result of tumor-derived humoral mediators.60,61,62
Pathology
Gross Presentation
Like undifferentiated carcinomas, SCCs are usually large (up to 8 cm), firm, tan-white masses, often involving one or both lobes of the thyroid gland. Additional nodules of tumor are frequently identified, and extrathyroidal extension is frequent. Necrosis is common (Fig. 15.12).41,52,56,57
Microscopic Description
Before making a diagnosis of a primary thyroid SCC, direct extension from the larynx or esophagus (clinically, radiographically, or operatively) must be excluded. A primary SCC is an invasive neoplasm with extensive destruction, composed entirely of squamous cells (Fig. 15.13).63 Vascular and perineural invasion, as well as extensive local extension, is common. The cells are cohesive and arranged in sheets, ribbons, and nests (Fig. 15.14) of pleomorphic cells with a variable nucleus-to-cytoplasm ratio (Fig. 15.15). Tumor cell spindling can be seen. Keratinization is frequently present, with keratin pearl formation (Fig. 15.15). Mitotic figures are common, including atypical forms. Tumors are graded as well differentiated, moderately differentiated, and poorly differentiated. They are also classified as keratinizing or nonkeratinizing; most thyroid tumors are poorly differentiated. An inflammatory infiltrate and stromal fibroplasia are frequently present (Fig. 15.16), sometimes in association with mucin production.41,44,46,49,52,56,58 It is important to note that papillary carcinoma and undifferentiated carcinoma can both exhibit areas of squamous differentiation,47,56,59,64,65,66,67,68,69,70 and these are much more common than in primary thyroid SCC. Fine needle aspiration may induce squamous metaplasia in the tumor.67 Therefore, although rare cases of SCC associated with follicular neoplasms have been reported,44,64,71 by definition and convention, a primary SCC of the thyroid gland must be a pure tumor without any other type of differentiation. If other differentiation is present, the primary tumor is diagnosed and coupled with a statement about squamous differentiation—for example, anaplastic carcinoma with squamous differentiation. Collision tumors (from different lobes) have been reported.72
Immunohistochemistry and Molecular Diagnostics
Pankeratin, CK19, and p63 are strongly positive within the neoplastic cells. CK7 and CK18 are focally immunoreactive, whereas epithelial membrane antigen (EMA) is occasionally reactive.25,53,57,70,73 CK1, CK4, CK6, CK10/13, CK20, and CD5 are nonreactive.57,73,74 S-100A9 is highlighted in a diffuse, laminated reaction that is different from the reaction seen with thymus-derived tumors.75 Diffusion artifacts may cause a falsepositive thyroglobulin reaction, but TTF1 immunoreactivity has been reported.53 The tumor cells usually demonstrate a high proliferation index with MIB-1 antibodies.25,41,53,56 Although only a few cases have been studied, abnormal p53 expression and loss of p21 expression have been reported.25,53,56,57 TP53 expression becomes greater as the neoplasm exhibits less squamous differentiation.57 Epidermal growth factor receptor (EGFR) may be overexpressed in these tumors (which showed gene polysomy), possibly providing a therapeutic alterative for these tumors.54,76
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree