Early detection of pulmonary or cardiogenic pulmonary disease (see Table 14.1)
Differential diagnosis of dyspnea
Presurgical assessment (e.g., ability to tolerate intraoperative anesthetics, especially during thoracic procedures)
Evaluation of risk factors for other diagnostic procedures
Detection of early respiratory failure
Monitoring progress of bronchopulmonary disease
Periodic evaluation of workers exposed to materials harmful to the respiratory system
Epidemiologic studies of selected populations to determine risks for or causes of pulmonary diseases
Workers’ compensation claims
Monitoring after pharmacologic or surgical intervention
TABLE 14.1 Conditions That Affect Ventilation | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Airway flow rates typically include measurements of instantaneous or average airflow rates during a maximal forced exhalation to assess airway patency and resistance. These tests also assess responses to inhaled bronchodilators or bronchial provocations.
Lung volumes and capacities measure the various air-containing compartments of the lung to assess air trapping (hyperinflation, overdistention) or reduction in volume. These measurements also help to differentiate obstructive from restrictive ventilatory impairments.
Gas exchange (diffusion capacity or transfer factor) measures the rate of gas transfer across the alveolar capillary membranes to assess the diffusion process. It can also monitor for side effects of drugs, such as bleomycin (antineoplastic) or amiodarone (antiarrhythmic), which can cause interstitial pneumonitis or pulmonary fibrosis. Diffusion capacity in the absence of lung disease (e.g., anemia) can also be evaluated.
Large capital letters denote primary symbols for gases: | ||
V = Gas volume | ||
[V with dot above] = Gas volume per unit time (the dot over the symbol indicates the factor per unit time, as in flow) | ||
P = Gas pressure or partial pressure of a gas in a gas mixture (exhaled air) or in a liquid (blood) | ||
F = Fractional concentration of a gas | ||
Small capital letters indicate the type of gas measured in relation to respiratory tract location or function: | ||
A = Alveolar gas | ||
D = Dead space gas | ||
E = Expired gas | ||
I = Inspired gas | ||
T = Tidal gas | ||
Chemical symbols for gases may be placed after the small capital letters: | ||
O2 = Oxygen | ||
CO = Carbon monoxide | ||
CO2 = Carbon dioxide | ||
N2 = Nitrogen | ||
Combinations of Symbols | ||
The following are some examples of the ways these symbols may be combined: | ||
FICO2 = Fractional concentration of inspired oxygen | ||
VT = Tidal volume | ||
VE = Volume of expired gas | ||
PACO = Partial pressure of carbon dioxide in alveolar gas | ||
Blood Gas Symbols | ||
Large capital letters are used as primary symbols for blood determinations: | ||
C = Concentration of a gas in blood | ||
S = Percent saturation of hemoglobin | ||
Q = Volume of blood | ||
Q = Volume of blood per unit time (blood flow) | ||
To indicate whether blood is capillary, venous, or arterial, lowercase letters are used: | ||
v = Venous blood | ||
a = Arterial blood | ||
c = Capillary blood | ||
s = Shunted blood | ||
bt = Brain tissue | ||
Blood gas symbols may be combined in the following ways: | |
PO2 = Oxygen tension or partial pressure of oxygen | |
PaO2 = Arterial oxygen tension or partial pressure of oxygen in arterial blood | |
PbtO2 = Brain tissue oxygen tension or partial pressure | |
PAO2 = Alveolar oxygen tension or partial pressure of oxygen in the alveoli | |
PCO2 = Carbon dioxide tension or partial pressure of carbon dioxide | |
PaCO2 = Partial pressure of carbon dioxide in arterial blood | |
PvCO2 = Partial pressure of carbon dioxide in venous blood | |
pH = Hydronium ion concentration | |
pHa = Hydronium ion concentration in arterial blood | |
SO2 = Oxygen saturation | |
SaO2 = Percent saturation of oxygen in arterial blood as measured by hemoximetry | |
(direct method) | |
SpO2 = Percent saturation of oxygen in arterial blood as determined by pulse oximetry | |
(indirect method) | |
SvO2 = Percent saturation of oxygen in venous blood | |
TCO2 = Total carbon dioxide content | |
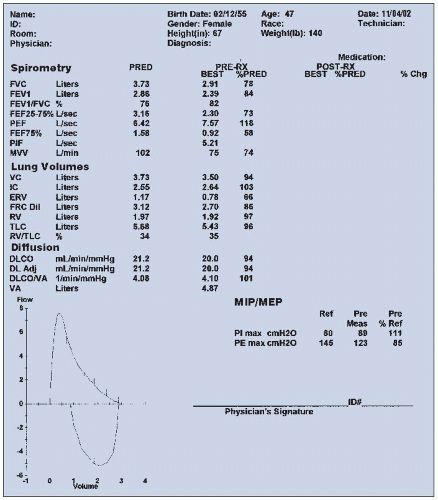
nebulizer, and the spirometry is repeated. Studies have shown a better bronchodilator response with combined drugs (e.g., albuterol plus ipratropium) than either alone. An increase in these values of 20% or more (>0.20) above the prebronchodilator level suggests a significant response to the bronchodilator and is consistent with a diagnosis of reversible obstructive airway disease (e.g., asthma). Persons with emphysema typically do not demonstrate this type of response to bronchodilator. Measured (actual) spirometry values are compared with predicted values by means of regression equations using age, height, weight, ethnicity, and gender and are expressed as a percentage of the predicted value. Typically, a value > 80% (>0.80) of predicted is considered within normal limits.
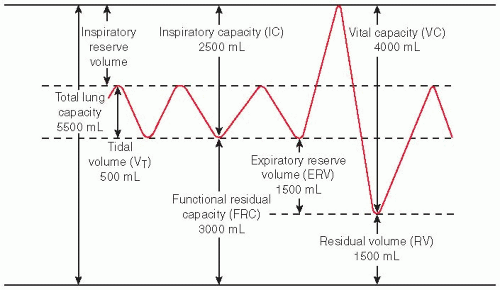
This list indicates terms used in measuring lung volumes and the units that express these measurements. | |
FVC = | Forced vital capacity: maximum amount of air that can be exhaled forcibly and completely after a maximal inspiration (liters) |
FEVt = | Forced expiratory volume at specific time intervals (e.g., 1, 2, and/or 3 seconds): volume of air expired during the first, second, third, etc., seconds of FVC maneuver (liters) |
FEVt/FVC = | Ratio of a timed forced expiratory volume to the forced vital capacity (e.g.,FEV1/FVC) (percent) |
FEF25-75 = | Forced expiratory flow between 25% and 75%: average flow of expired air measured between 25% and 75% of the FVC maneuver (liters/second) |
PEFR = | Peak expiratory flow rate: maximum flow of expired air attained during an FVC maneuver (liters/second or liters/minute) |
PIFR = | Peak inspiratory flow rate: maximum flow of inspired air achieved during a forced maximal inspiration (liters/second or liters/minute) |
FEF25 = | Forced instantaneous expiratory flow rate at 25% of lung volume achieved during an FVC maneuver (liters/second or liters/minute) |
FEF50 = | Forced instantaneous expiratory flow rate at 50% of lung volume achieved during an FVC maneuver (liters/second or liters/minute) |
FEF75 = | Forced instantaneous expiratory flow rate at 75% of lung volume achieved during an FVC maneuver (liters/second or liters/minute) |
FRC = | Functional residual capacity: volume of air remaining in the lung at the end of a normal expiration (i.e., end-tidal expiration) (liters) |
IC = | Inspiratory capacity: maximum amount of air that can be inspired from end-tidal expiration (liters) |
IRV = | Inspiratory reserve volume: maximum amount of air that can be inspired from end-tidal inspiration (liters) |
ERV = | Expiratory reserve volume: maximum amount of air that can be expired from end-tidal expiration (liters) |
RV = | Residual volume: volume of gas left in the lung after a maximal expiration (liters) |
VC = | Vital capacity: maximum volume of air that can be expired after a maximal inspiration (liters) |
TLC = | Total lung capacity: volume of gas contained in the lungs after a maximal inspiration (liters) |
DLCO = | Carbon monoxide diffusing capacity of the lung: rate of diffusion of carbon monoxide across the alveolar capillary membrane (i.e., rate of gas transfer across the alveolar capillary membrane) (milliliters/minute per millimeter of mercury) |
CV = | Closing volume: volume at which the lower lung zones cease to ventilate, presumably as a result of airway closure (percent of vital capacity) |
MVV = | Maximum voluntary ventilation: maximum number of liters of air a patient can breathe per minute by a voluntary effort (liters/minute) |
VISOV = | Volume of isoflow: volume for which flow is the same with air and with helium during an FVC maneuver (percent) |
This list shows some of the other symbols found in this chapter. | |
f = | = Frequency (of breathing) |
CL = | Compliance of the lung |
COHb = | Carboxyhemoglobin |
DLO2 = | Oxygen diffusing capacity of the lung |
A-aDO2 = | = Alveolar-to-arterial oxygen gradient |
BSA = | Body surface area (square meters) |
H2CO3 = | Carbonic acid |
HCO3– = | Bicarbonate ion |
TGV = | Thoracic gas volume (also expressed as VTG) |
Raw = | Airway resistance |
Gaw = | Airway conductance |
F-V = | Flow-volume |
V-T = | Volume-time |
Have the patient either sit or stand. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
Ask the patient to take a maximal inspiration and then forcibly and completely exhale into the spirometer.
Have the patient repeat this maneuver a minimum of three times. The two best tracings should compare within ±200 mL of one another, or additional forced expiratory efforts will be needed.
Administer bronchodilators with a handheld nebulizer, and repeat spirometry if indicated.
See Chapter 1 guidelines for intratest care.
 PROCEDURAL ALERT
PROCEDURAL ALERT
Before testing, assess the patient’s ability to comply with breathing requirements.
The patient may experience lightheadedness, shortness of breath, or other slight discomforts. These symptoms are generally transitory. An appropriate rest period is usually all that is needed. If symptoms persist, testing is terminated.
Rarely, momentary loss of consciousness (caused by anoxia during forced expiration) may occur. Follow established protocols for testing this.
Assess for contraindications such as pain or altered mental status.
With obstructive ventilatory impairments such as asthma, airway collapse occurs during the forced expiratory effort. This leads to decreases in airway flow rates and also, in the more severe forms, to apparent loss of volumes. Obstructive ventilatory impairments include the following:
Emphysema
Bronchitis
Asthma
Cystic fibrosis
Byssinosis (exposure to cotton dust)
With restrictive ventilatory impairments, the FVC is reduced; however, flow rates can be normal or elevated. Restrictive ventilatory impairments include the following:
Pulmonary fibrosis
Lung resection
Thoracic cage deformities (e.g., pectus excavatum, kyphoscoliosis)
Asbestosis (exposure to the asbestos fiber)
Silicosis (exposure to crystalline silica dust)
Bronchodilators (e.g., albuterol) should be withheld for at least 4 hours if tolerated.
Respiratory infections may decrease airflow during the maneuver.
Patient noncompliance can adversely affect the results because this test is effort dependent.
Explain the purpose and procedure of the spirometry test. Explain that the patient will be asked to perform a maximal forced inspiration in addition to the forced expirations.
Remind the patient that a light meal may be eaten before the test. However, no caffeine should be taken before testing. Specific instructions will be given regarding the use of bronchodilators or inhaler medications before the test.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Evaluate for dizziness, shortness of breath, or chest discomfort. Usually, these symptoms are transitory and subside after a short rest. If symptoms persist, use established follow-up protocols.
Treatment of pulmonary disorders includes bronchodilators, corticosteroids, supplemental oxygen, and surgery. In patients with cystic fibrosis, drugs (e.g., dornase sulfa [Pulmozyme]) can be used to thin secretions (i.e., reduce sputum viscosity).
See Chapter 1 guidelines for safe, effective, informed posttest care.
Have the patient either sit or stand. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
Ask the patient to take a maximal inspiration forcibly and completely exhale into the spirometer and then inspire forcibly and completely again.
Have the patient repeat this maneuver a minimum of three times. Report the highest value.
The PIFR can also be measured with a handheld peak flow meter.
PIFR is reduced in neuromuscular disorders, with weakness or poor effort, and in extrathoracic airway obstruction (i.e., substernal thyroid, tracheal stenosis, and laryngeal paralysis).
The PIFR is decreased in upper airway obstruction.
Poor patient effort compromises the test.
Inability to maintain an airtight seal around the mouthpiece
Explain the purpose and procedure of the test. Assess the patient’s ability to comply.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Have the patient either sit or stand. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
Ask the patient to take a maximal inspiration forcibly and completely exhale into the spirometer and then inspire forcibly and completely again.
Have the patient repeat this maneuver a minimum of three times. Report the highest value.
The PEFR can also be measured with a handheld peak flow meter.
The PEFR usually is decreased in obstructive disease (e.g., emphysema), during acute exacerbations of asthma, and in upper airway obstruction (e.g., tracheal stenosis).
The PEFR usually is normal in restrictive lung disease but is reduced in severe restrictive situations.
Poor patient effort compromises the test.
Inability to maintain an airtight seal around the mouthpiece
Explain the purpose and procedure of the test. Assess the patient’s ability to comply.
See Chapter 1 guidelines for safe, effective, informed pretest care.
Monitor patient for dizziness, lightheadedness, or chest pain following the test. Generally, these symptoms are transient and will subside quickly. If not, follow established protocols.
See aftercare guidelines for volume-time spirograms on page 921.
See Chapter 1 guidelines for safe, effective, informed posttest care.
Total lung capacity (TLC)
Tidal volume (VT)
Inspiratory capacity (IC)
Inspiratory reserve volume (IRV)
Residual volume (RV)
Functional residual capacity (FRC)
Expiratory reserve volume (ERV)
Vital capacity (VC)
Fit the patient with nose clips. Instruct the patient to breathe normally through the mouthpiece/ filter (bacterial/viral) combination that is attached to the lung volume apparatus. The patient is generally in the seated position.
There are three methods, depending on the instrumentation used:
Nitrogen washout or open-circuit technique
Helium dilution or closed-circuit technique
Whole-body plethysmography
Have the patient breathe normally for about 3 to 7 minutes.
Perform the test a second time. The FRC should vary by not more than 5% to 10% (0.05 to 0.10). Report the average of the test values.
See Chapter 1 guidelines for intratest care.
A value <75% (<0.75) of the predicted is consistent with restrictive ventilatory impairment.
A value >125% (>1.25) of predicted demonstrates air trapping (hyperinflation), consistent with obstructive airway disease (e.g., emphysema, asthma, bronchiolar obstruction).
Explain the purpose and procedure of the test. Explain that this is a noninvasive test requiring patient cooperation. Assess the patient’s ability to comply.
Record the patient’s age, gender, weight, and height.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Explain test outcomes; allow the patient to rest if necessary.
See Chapter 1 guidelines for safe, effective, informed posttest.
Remember that the RV is determined indirectly from other tests; that is, it is mathematically derived by subtracting the measured expiratory reserve volume (ERV) from the FRC.
See Chapter 1 guidelines for intratest care.
An increase in the RV (>125% [>1.25] of predicted) indicates that, despite a maximal expiratory effort, the lungs still contain an abnormally large amount of gas (air trapping). This type of change occurs in young asthmatic patients and usually is reversible. In emphysema, the condition is permanent.
Increased RV is characteristic of emphysema, chronic air trapping, and chronic bronchial obstruction.
The RV and the FRC usually increase together, but not always.
The RV sometimes decreases in diseases that occlude many alveoli.
An RV <75% (<0.75) of predicted is consistent with restrictive disorders (e.g., interstitial pulmonary fibrosis).
Explain the purpose of the test and how the results are calculated.
See Chapter 1 guidelines for safe, effective, informed pretest care.
Interpret test results and monitor as necessary.
Follow Chapter 1 guidelines for safe, effective, informed posttest care.
Have the patient either sit or stand. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
Ask the patient to exhale completely and resume normal breathing. Record results on graph paper.
Ask the patient to repeat this maneuver at least twice. The measured volumes should be within ±60 mL of one another. Report the average value.
A decreased ERV indicates a chest wall restriction resulting from nonpulmonary causes.
Decreased values are associated with an elevated diaphragm (e.g., massive obesity, ascites, pregnancy). Decreased values also occur with massive enlargement of the heart, pleural effusion, kyphoscoliosis (abnormal curvature of the spine), or thoracoplasty (removal of one or more ribs).
Decreases in ERV also are seen in obstruction resulting from an increase in the RV impinging on the ERV.
Explain the purpose and procedure of the spirometry test. Inform the patient that the test is noninvasive. Assess the patient’s ability to comply with test procedures.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Interpret test outcomes and counsel about respiratory abnormalities.
Follow Chapter 1 guidelines for safe, effective, informed posttest care.
Have the patient either sit or stand. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
After several breaths, ask the patient to inhale maximally, expanding the lungs as much as possible from end-tidal expiration. Have the patient resume normal breathing. Record the results on graph paper.
Repeat step 2 two or more times until the two best values are within 5% of each other. Select the largest inspired volume value.
Changes in the IC usually parallel increases or decreases in the vital capacity (VC).
Decreases in IC can be related to either restrictive or obstructive ventilatory impairments.
Instruct the patient about the purpose and procedure of the test and the need for patient cooperation.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Interpret test outcomes and monitor the patient.
See Chapter 1 guidelines for safe, effective, informed posttest care.
Have the patient either sit or stand. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
Instruct the patient then to inhale as deeply as possible and then to exhale completely, with no forced or rapid effort.
Record results on graph paper.
Repeat the procedure until the measurements are within about 5% of each other.
A reduced VC is defined as a value <80% (<0.80) of predicted.
The VC can be lower than expected in either a restrictive or an obstructive disorder. Inadequate patient effort causes lower VC values.
A decreased VC can be related to depression of the respiratory center in the brain, neuromuscular diseases, pleural effusion, pneumothorax, pregnancy, ascites, limitations of thoracic movement, scleroderma (autoimmune disease resulting in fibrosis), kyphoscoliosis, or tumors.
The VC increases with physical fitness and greater height.
The VC decreases with age (after age 30 years).
The VC is generally less in women than in men of the same age and height.
The VC is decreased by approximately 15% in African Americans and by 20% to 25% in Asians compared with Caucasians of the same age, height, and gender.
Explain the purpose and procedure of the test and need for patient cooperation. Assess for interfering factors.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Interpret outcomes, monitor patient signs and symptoms, and follow up if necessary.
See Chapter 1 guidelines for safe, effective, informed posttest care.
Have the patient either sit or stand. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
Ask the patient to inspire maximally and exhale maximally. The total amount of air exhaled is the VC.
Use the following formula to derive the TLC mathematically: TLC = VC + RV.
Increased values are associated with:
Emphysema
Cystic fibrosis
Hyperinflation
Decreased values are associated with:
Pulmonary edema
Atelectasis (collapse of part of the lung)
Neoplasms
Pulmonary congestion
Pneumothorax
Thoracic restriction
Explain the purpose and procedure of the test. Even though it is noninvasive, it does require patient effort and cooperation.
See Chapter 1 guidelines for safe, effective, informed pretest care.
Have the patient either sit or stand. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the diffusion instrument.
Ask the patient to expire maximally and then inspire maximally (a diffusion gas mixture), hold breath for 10 seconds, and then exhale, at which time a sample of exhaled gas is obtained.
Two techniques are used by laboratories:
Single-breath or breath-holding technique
Steady-state technique
See Chapter 1 guidelines for intratest care.
Decreased values are associated with:
Multiple pulmonary emboli
Emphysema
Lung resection
Pulmonary fibroses:
Sarcoidosis (abnormal collection of inflammatory cells in multiple organs)
Systemic lupus erythematosus (SLE)
Asbestosis
Pneumonia
Anemia
Increased levels of carboxyhemoglobin (COHb)
Pulmonary resection
Scleroderma
Increased values are observed in polycythemia, left-to-right shunts, pulmonary hemorrhage, and exercise.
The value is relatively normal in chronic bronchitis.
Explain the purpose and procedure. Assess for interfering factors and explain that this noninvasive test requires patient cooperation. Assess the patient’s ability to comply.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Have the patient either sit or stand. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
Instruct the patient to breathe into the spirometer as deeply and rapidly as possible for 10 to 15 seconds. Usually, the frequency reaches 40 to 70 breaths per minute, and the tidal volumes are about 50% of the vital capacity (VC).
The actual values are extrapolated from the 10- to 15-second time interval to a 1-minute time period.
Typically, the maneuver is performed twice and the largest value is reported.
Obstructive ventilatory impairments of moderate to severe degree, abnormal neuromuscular control, and poor patient effort are causes of low values.
In restrictive disease, the value is usually normal; however, in more severe forms, MVV may be decreased.
Explain the purpose and procedure of the test. Explain that it is a noninvasive test that requires patient cooperation. Assess the patient’s ability to comply.
Record the patient’s age, height, and gender.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Instruct the patient, who should be in a seated position and wearing a nose clip, to inspire maximally. Place the mouthpiece of the handheld pressure manometer into the mouth and have the patient perform a forced expiration. Record this maximal sustained (1 to 3 seconds) pressure against the internal occlusion of the manometer as the MEP.
Repeat this same procedure to obtain the MIP, except that this time, the patient fully exhales before placing the mouthpiece of the manometer in the mouth. Have the patient then inspire forcefully, and record the maximal sustained (1 to 3 seconds) pressure.
Repeat each procedure, and record the best of three measurements for each.
See Chapter 1 guidelines for intratest care.
Decreases in both MEP and MIP are seen in neuromuscular disorders (e.g., myasthenia gravis, poliomyelitis).
Decreased MEP is common in both severe obstructive disease (e.g., emphysema) and severe restrictive ventilatory impairment (e.g., interstitial pulmonary fibrosis).
Decreased MIP is observed in patients with chest wall abnormalities (e.g., kyphoscoliosis) and in hyperinflation (e.g., emphysema).
Explain the purpose and procedure of the test. Explain that it is a noninvasive, effort-dependent maneuver that requires patient cooperation.
Record the patient’s age and sex.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Have the patient assume a seated position. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
Ask the patient to exhale completely, to inhale 100% O2, and then to exhale completely at the rate of approximately 0.5 L/second.
During exhalation, monitor simultaneously both the expired volume and percentage of alveolar nitrogen on an X-Y recorder. Remember that a sudden increase in nitrogen represents the closing volume.
Values are increased for those conditions in which the airways are narrowed (e.g., bronchitis, early airway obstruction, chronic smoking, old age).
A change in the slope of the nitrogen curve of >2% is indicative of maldistribution of inspired air (i.e., uneven alveolar ventilation).
Congestive heart failure, with subsequent edema, may also contribute to decreasing patency of the small airways leading to an increase in the CV.
The CV increases with age.
Patients in congestive heart failure may show an increased CV.
Explain the purpose and procedure of the test. Explain that this is a noninvasive test that requires patient cooperation. Assess the patient’s ability to comply with breathing requirement and instructions. Assess for interfering factors.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Explain the meaning of test outcomes and possible need for follow-up testing and treatment of early small airway disease.
See Chapter 1 guidelines for safe, effective, informed posttest care.
Have the patient assume a seated position. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
Have the patient perform a baseline F-V loop, which is recorded by a spirometer on an X-Y recorder.
Have the patient next breathe a mixture of 80% He and 20% O2 for several breaths and then perform another F-V loop maneuver; this is the HeliOx F-V loop.
Superimpose the F-V loop tracings, and measure the volume of isoflow at the point at which the two loops intersect.
Explain the purpose and procedure of the test.
Follow guidelines in Chapter 1 regarding safe, effective, informed pretest care.
Interpret test outcomes and possible need for follow-up testing and treatment.
See Chapter 1 guidelines for safe, effective, informed posttest care.
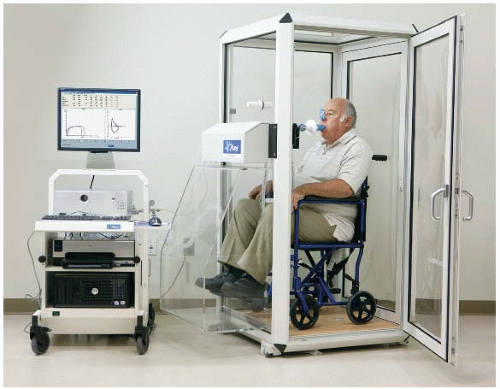
Have the patient sit in the plethysmograph (body box). Fit with nose clips, and have the patient breathe through a mouthpiece/filter (bacterial/viral) combination connected to a transducer (Fig. 14.4).
Ensure that the body box door is secured. Delay the test for a few minutes to allow the box pressure to stabilize due to temperature changes.
Instruct the patient to perform a panting maneuver while holding the cheeks rigid and the glottis open against a closed shutter located within the transducer assembly. Plethysmograph box and mouth pressures are displayed on a monitor for subsequent determination of the VTG.
Next, ask the patient to breathe rapidly and shallowly. Plethysmograph box pressure changes versus flow are displayed on a monitor for subsequent determination of the Raw.
To determine CL, pass a balloon catheter through the nose into the patient’s esophagus. Typically, the balloon catheter is lightly coated with a topical anesthetic (e.g., lidocaine jelly) for patient comfort. Ensure that the inflated balloon is connected to a transducer, and instruct the patient to breathe normally. Changes in intraesophageal pressure during normal respiration (which mimic changes in intrapleural pressure) are recorded for determination of the CL.
See Chapter 1 guidelines for intratest care.
An increased VTG demonstrates air trapping, consistent with obstructive pulmonary disease, for example, emphysema.
An increased Raw or decreased Gaw demonstrates increased resistance to airflow through the tracheobronchial tree; this is seen in asthma, emphysema, bronchitis, and other forms of obstruction.
An increase in CL (i.e., lung is more distensible) is seen in obstructive diseases.
A decrease in CL (i.e., lung is more stiff) is seen in fibrotic diseases, restrictive diseases, pneumonia, congestion, and atelectasis.
Explain the purpose and procedure of the test.
Assure the patient that although the chamber is airtight, the test only takes a few minutes. A technician will be in constant attendance to open the door should that be necessary. There is also a handle inside of the body box should the patient feel anxious and need to open the door. Assess for ability to comply with test requirements and instructions. Tactfully assess for predisposition to claustrophobia, panic attacks, or other similar responses.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Allow the patient time to rest quietly if necessary.
Explain the meaning of test outcomes.
See Chapter 1 guidelines for safe, effective, informed posttest care.
Have the patient assume the seated position. Place nose clips on the nose and instruct the patient to breathe normally through a mouthpiece/filter (bacterial/viral) combination into the spirometer.
Have the patient perform a forced expiratory maneuver, and measure and record the baseline FEV1 (or Raw measurement).
The patient will inhale increasing concentrations of methacholine chloride (0.062- 16.00 mg/mL) or histamine by nebulizer. Repeat the FVC or Raw maneuver after each successive concentration is inhaled. A 20% reduction in the FEV1 (primary outcome variable) or 35% increase in Raw is considered a positive response.
Administer an inhaled bronchodilator when or if a decrease of >20% from baseline is reached.
If a patient goes through all dilution ratios and a 20% reduction in the FEV1 or >35% increase in Raw is not reached, the test is considered negative.
Remember that if the methacholine causes no change, histamine testing may be ordered.
See Chapter 1 for guidelines for intratest care.
Explain the purpose and procedure of the test and the need for patient cooperation. Assess the patient’s ability to comply.
Withhold bronchodilators for 8 hours and antihistamines for 48 hours before testing, if tolerated.
Follow Chapter 1 guidelines for safe, effective, informed pretest care.
Explain the meaning of test outcomes.
If the test is positive, advise the patient to avoid antigens that may be causing hypersensitivity reactions and bronchospasms.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree