Principles of Infectious Diseases Epidemiology
Lennox K. Archibald
Epidemiology is defined as the study of the factors determining the occurrence of diseases in human populations. It is an indispensable tool for characterizing infectious disease occurrences in medical institutions, communities, regions, or industry, and for determining the exposure-disease relationship in humans and the modes of acquisition and spread that are critical for treatment, control, and prevention of these infectious disease occurrences. Clinicians, microbiologists, and other personnel involved in the preventive and public health professions use epidemiologic methods for disease surveillance, outbreak investigations, infectious diseases outcome measurements, and observational studies to identify risk factors for various infectious diseases. Knowledge of these risk factors is essential for making decisions regarding further epidemiologic or microbiological investigations, directing research activities, implementing relevant prevention and control measures or interventions, and establishing public health policies. In the pharmaceutical and biomedical industries, the application of epidemiologic methods is integral to the investigation of intrinsic contamination of products, ascertainment and characterization of risk factors for contamination, and maintenance of quality assurance practices in the laboratory or manufacturing operations before distribution of products.
The use of epidemiology and the use of statistical methods to analyze epidemiologic data grew out of attempts to understand, predict, and control the great epidemics of our past; the diseases associated with those early epidemics were largely infectious. The study and implementation of infection control practices and interventions grew out of the need to understand and control the institutional epidemics of infectious diseases that complicate the care of the ill (1,2). Thus, discussions of the principles of epidemiology begin with examples of methods that were first formalized in the study of transmissible microorganisms, many of which continue to cause problems today.
The term hospital epidemiology was a modern addition by workers in the United States (3), as was the recognition of the potential use of epidemiologic methods in hospitals for the study and control of noninfectious diseases (4). The term nosocomial infection has traditionally defined acute infections acquired in the hospital inpatient setting (5). However, in the current era of managed care, healthcare systems in the United States have evolved from the traditional acute care hospital inpatient setting to a new integrated, extended care model that now encompasses hospitals, outpatient clinics, ambulatory centers, longterm care facilities, and the home. As expected, infections (and antimicrobial resistance among implicated pathogens) may be acquired at any of these levels of care. For this reason, the term nosocomial infection has been replaced by healthcare-associated infection. Except for the acute care hospitals, however, the relative importance of each of these levels of care as risk factors for the acquisition of healthcare-associated infections remains largely uncharacterized or unknown.
The terms hospital epidemiology and infection control remain synonymous in the minds of many, and both the terms and their associated programs have grown in definition and function over the past five decades. Interest in infection control has broadened from focused concerns
with puerperal sepsis and surgical site infection to full, scientifically tested programs of surveillance, prevention, and control of healthcare-associated infections acquired at other anatomic sites. Hospital epidemiology programs were among the earliest projects used to demonstrate the utility of the scientific method and statistics for characterizing and analyzing infectious diseases data and using the results of these analyses to improve the quality of care and patient outcomes. In the special environment of the acute care hospital, a natural repetition of earlier studies of population-based infectious diseases provided the basis for epidemiologic investigations.
with puerperal sepsis and surgical site infection to full, scientifically tested programs of surveillance, prevention, and control of healthcare-associated infections acquired at other anatomic sites. Hospital epidemiology programs were among the earliest projects used to demonstrate the utility of the scientific method and statistics for characterizing and analyzing infectious diseases data and using the results of these analyses to improve the quality of care and patient outcomes. In the special environment of the acute care hospital, a natural repetition of earlier studies of population-based infectious diseases provided the basis for epidemiologic investigations.
Surveillance data generated from epidemiologic studies may be used to determine the need for clinical or public health action; assess the effectiveness of prevention, intervention, or control programs, or diagnostic algorithms; or set priorities for rational or appropriate use of limited microbiology resources, planning, and research. An understanding of epidemiology is important for quantifying and interpreting microbiology and pharmaceutical data, and for application of these data to clinical practice, quality assurance, hypothesis generation during investigation of outbreaks and other adverse events, rational prescribing policies, and public health.
Data from epidemiologic and microbiological studies can inform diagnostic and therapeutic practice and indicate areas for allocation of already scarce resources. For example, one of the perennial problems that clinicians and microbiologists face is how to differentiate between true bacteremia and blood culture contamination resulting from coagulase-negative staphylococci, which are the most frequently isolated microorganisms in blood cultures (6). Blood culture contamination can occur during venipuncture if the skin is not adequately cleaned, after the blood draw at the time of inoculation of blood into the culture bottle, or at some point during processing of blood culture bottles in the microbiology laboratory. To make an informed decision on true bacteremia versus contamination, clinicians and microbiologists need to be familiar with the epidemiology of bloodstream infections in different clinical settings and be able to integrate these data with the relevant clinical and microbiology information at hand so that a decision could be made whether or not to initiate antimicrobial therapy or request additional, supplemental investigations that might facilitate the decision-making process.
DEFINITIONS
In the application and discussion of epidemiologic principles, standard definitions and terminology have been widely accepted (7,8). The definitions of some commonly used terms are outlined in this section:
Attack rate A ratio of the number of new infections divided by the number of exposed, susceptible individuals in a given period, usually expressed as a percentage. Other terms are the incidence rate and the case rate.
Attributable mortality indicates that an exposure was a contributory cause of or played an etiologic role leading to death.
Attributable risk The measure of impact of a causative factor. The attributable risk establishes how much of the disease or infection is attributable to exposure to a specific risk factor. It is a proportion where the numerator is the difference between the incidence in exposed and unexposed groups and the denominator is the incidence for the exposed group.
Bias The difference between a true value of an epidemiologic measure and that which is estimated in a study. Bias may be random or systematic. There are three types of bias: selection bias, information bias, and confounding. Selection bias is a distortion in the estimate of effect resulting from the manner in which parameters are selected for the study population. Information bias depends on the accuracy of the information collected. Confounding arises from unrecognized factors that may affect interpretation of epidemiologic data. Unrecognized, systematic bias presents the greatest danger in studies by suggesting relationships that are not valid (see also Chapter 2).
Carrier An individual (host) who harbors a microorganism (agent) without evidence of disease and, in some cases, without evidence of host immune response. This carriage may take place during the latent phase of the incubation period as a part of asymptomatic disease or may be chronic following recovery from illness. Carriers may shed microorganisms into the environment intermittently or continuously, and this shedding may lead to transmission. Shedding and potential transmission may be increased by other factors affecting the host, including infection by another agent.
Case An individual in a population or group recognized as having a particular disease or condition under investigation or study. This definition may not be the same as the clinical definition of a case.
Case-fatality rate A ratio of the number of deaths from a specific disease divided by the number of cases of disease, expressed as a percentage.
Cluster An aggregation of relatively uncommon events or diseases in time and/or space in numbers that are believed to be greater than are expected by chance alone.
Colonization The multiplication of a microorganism at a body site or sites without any overt clinical expression or detected immune reaction in the host at the time that the microorganism is isolated. Colonization may or may not be a precursor of infection. Colonization may be a form of carriage and is a potential source of transmission.
Communicability The characteristic of a human pathogen that enables it to be transmitted from one person to another under natural conditions. Infections may be communicable or noncommunicable. Communicable infections may be endemic, epidemic, or pandemic.
Communicable period The time in the natural history of an infection during which transmission to susceptible hosts may take place.
Confounding An illusory association between two factors when in fact there is no causal relationship between the two. The apparent association is caused by a third variable that is both a risk factor for the outcome or disease
and is associated with but not a result of the exposure in question.
and is associated with but not a result of the exposure in question.
Contact An exposed individual who might have been infected through transmission from another host or the environment.
Contagious Having the potential for transmission.
Contamination The presence of an agent (e.g., microorganism) on a surface or in a fluid or material—therefore, a potential source for transmission.
Cumulative incidence The proportion of at-risk persons who become diseased during a specified period of time.
Endemic The usual level or presence of an agent or disease in a defined population during a given period.
Epidemic An unusual, higher-than-expected level of infection or disease by an agent in a defined population in a given period. This definition assumes previous knowledge of the usual, or endemic, levels.
Epidemic curve A graphic representation of the distribution of defined cases by the time of onset of their disease.
Epidemic period The time period over which the excess cases occur.
Hyperendemic The level of an agent or disease that is consistently present at a high incidence and/or prevalence rate.
Immunity The resistance of a host to a specific agent, characterized by measurable and protective surface or humoral antibody and by cell-mediated immune responses. Immunity may be the result of specific previous experience with the agent (wild infection), from transplacental transmission to the fetus, or from active or passive immunization to the agent. Immunity is relative and governed through genetic control. Immunity to some agents remains throughout life, whereas for others, it is short-lived, allowing repeat infections by the same agent. Immunity may be reduced in extremes of age, through disease, or through immunosuppressive therapy.
Immunity: cell-mediated versus humoral Cell-mediated immune protection, largely related to specific T-lymphocytic activity, as opposed to humoral immunity, which is measured by the presence of specific immunoglobulins (antibodies) in surface body fluids or circulating in noncellular components of blood. Antibodies are produced by B lymphocytes, also now recognized to be under the influence of T-lymphocytic functions.
Immunogenicity An agent’s (microorganism’s) intrinsic ability to trigger specific immunity in a host. Certain agents escape host defense mechanisms by intrinsic characteristics that fail to elicit a host immune response. Other agents evoke an immune response that initiates a disease process in the host that increases cellular damage and morbidity beyond the direct actions of the microorganism itself. These disease processes may continue beyond the presence of living microorganisms in the host.
Incidence The ratio of the number of new infections or disease in a defined population in a given period to the number of individuals at risk in the population. “At risk” is frequently defined as the number of potentially exposed susceptible persons. Incidence is a measure of the transition from a nondiseased to a diseased state and is usually expressed as numbers of new cases per thousands (1,000, 10,000, or 100,000) per year.
Incidence rate or density Similar to the incidence but members of the at-risk population may be followed for different lengths of time. Thus, the denominator is the sum of each person’s time at risk (i.e., total person-time of observation).
Incubation period The period between exposure to an agent and the first appearance of evidence of disease in a susceptible host. Incubation periods are typical for specific agents and may be helpful in the diagnosis of unknown illness. Incubation periods may be modified by extremes of dose or by variations in host immune function. The first portion of the incubation period following colonization and infection is frequently a silent period, called the latent period. During this time, there is no evidence of host response(s) and evidence of the presence of the infecting agent may not be measurable. However, transmission of the microorganism to other hosts, though reduced during this period, is a recognized risk (e.g., chicken pox, hepatitis B virus, human immunodeficiency virus [HIV]). Measurable early immune responses in the host may appear shortly before the first signs and symptoms of disease, marking the end of the latent period. Signs and symptoms of disease commonly appear shortly thereafter, marking the end of the incubation period.
Index case The first case to be recognized in a series of transmissions of an agent in a host population. In semiclosed populations, as typified by chronic disease hospitals, the index case may first introduce an agent not previously active in the population.
Infection The successful transmission of a microorganism to the host with subsequent multiplication, colonization, and invasion. Infection may be clinical or subclinical and may not produce identifiable disease. However, it is usually accompanied by measurable host response(s), either through the appearance of specific antibodies or through cell-mediated reaction(s) (e.g., positive tuberculin test results). An infectious disease may be caused by the intrinsic properties of the agent (invasion and cell destruction, release of toxins) or by associated immune response in the host (cell-mediated destruction of infected cells, immune responses to host antigens similar to antigens in the agent).
Infectivity The characteristic of the microorganism that indicates its ability to invade and multiply in the host. It is frequently expressed as the proportion of exposed patients who become infected.
Isolation The physical separation of an infected or colonized host, including the individual’s contaminated body fluids and environmental materials, from the remainder of the at-risk population in an attempt to prevent transmission of the specific agent to the latter group. This is usually accomplished through individual environmentally controlled rooms or quarters, hand washing following contact with the infected host and environment, and the use of barrier protective devices, including gowns, gloves, and, in the case of airborne agents, an appropriate mask.
Morbidity rate The ratio of the number of persons infected with a new clinical disease to the number of persons at risk in the population during a defined period; an incidence rate of disease.
Mortality rate The ratio of those infected who have died in a given period to the number of individuals in the defined population. The rate may be crude, related to all causes, or disease-specific, related or attributable to a specific disease in a population at risk for the disease.
Odds The ratio of the probability of an event occurring to the probability of it not occurring.
Pandemic An epidemic that spreads over several countries or continents and affects many people.
Pathogenicity The ability of an agent to cause disease in a susceptible host. The pathogenicity of a specific agent may be increased in a host with reduced defense mechanisms. For some agent-host interactions, the resultant disease is due to the effects of exaggerated or prolonged action of defense mechanisms of the host.
Prevalence The ratio of the number of individuals measurably affected or diseased by an agent in a defined population at a particular point in time. The proportion of the population having the disease during a specified time period, without regard to when the process or disease began, defines the period prevalence.
Pseudo-outbreak Real clustering of false infections or artifactual clustering of real infections. Often it is identified when there is increased recovery of unusual microorganisms.
Rate An expression of the frequency with which an event occurs in a defined population. All rates are ratios. Some rates are proportions; that is, the numerator is a part of the denominator. A comparable rate is a rate that controls for variations in the distribution of major risk factors associated with an event.
Ratio An expression of the relationship between a numerator and a denominator where the two are usually distinct and separate quantities, neither being a part of the other.
Relative risk The ratio of the incidence rate of infection in the exposed group to the incidence rate in the unexposed group. Used to measure the strength of an association between exposures or risk factors and disease.
Reservoir Any animate or inanimate niche in the environment in which an infectious agent may survive and multiply to become a source of transmission to a susceptible host. Medical care workers and patients constitute the main animate reservoir for microorganisms associated with healthcare-associated infections; waterrelated sources are important inanimate reservoirs that have been implicated in outbreaks related to dialysis units and to air conditioning systems.
Secular trend Profile of the changes in measurable events or in the incidence rate of infection or disease over an extended period of time; also called a temporal trend.
Sensitivity For surveillance systems, the ratio of the number of patients reported to have an infection divided by the number of patients who actually had an infection.
Specificity For surveillance systems, the ratio of the number of patients who were reported not to have an infection divided by the number of patients who actually did not have an infection.
Sporadic Occurring irregularly and usually infrequently over a period of time.
Surveillance The ongoing systematic collection, analysis, and interpretation of healthcare data essential to the planning, implementation, and evaluation of public health practice, closely integrated with the timely dissemination of these data to those contributing data or to other interested groups who need to know. Surveillance was popularized by Langmuir and others at the Centers for Disease Control and Prevention (CDC) and has been the basic method in infection control programs in the United States since the 1960s.
Susceptibility A condition of the host that indicates absence of protection against infection by an agent. This is usually marked by the absence of specific antibodies or specific measures of cell-mediated immunity against the infecting microorganism.
Transmission The method by which any potentially infecting agent is spread to another host. Transmission may be direct or indirect. Direct transmission may take place by touching between hosts, by the projection of large droplets in coughing and sneezing onto another host, or by direct contact by a susceptible host with an environmental reservoir of the agent. Indirect transmission may be vehicle-borne, airborne, or vector-borne. In vehicle-borne transmission, contaminated environmental sources, including water, food, blood, and laundry, may act as an intermediate source of an infectious agent for introduction into a susceptible host. The agent may have multiplied or undergone biologic development in the vehicle. In airborne transmission, aerosols containing small (1-5 µm) particles may be suspended in air for long periods and inspired into the lower respiratory tract to become a site of infection in a host. These infectious particles may be generated by evaporation of larger particles produced in coughing and sneezing (Mycobacterium tuberculosis), by mechanical respiratory aerosolizers (Legionella), or by wind or air currents (fungal spores). In vector-borne transmission, arthropods or other invertebrates may carry or transmit microorganisms, usually through inoculation by biting or by contamination of food or other materials. The vector may be infected itself or act only as a mechanical carrier of the agent. If the vector is infected, the agent may have multiplied or undergone biologic development in the vector. This type of transmission has been of little importance for healthcare-associated infections in the United States.
Virulence The intrinsic capabilities of an agent to infect a host and produce disease and a measure of the severity of the disease produced. In the extreme, this is represented by the number of patients with clinical disease who develop severe illness or die—the case-fatality rate.
EPIDEMIOLOGIC METHODS APPLIED TO INFECTIOUS DISEASES
The classic epidemiologic methods are essential for the study, characterization, and understanding of the various infections that occur in healthcare settings, communities,
or regions. Such methods are used to determine the exposure-disease relationship in humans; establish the modes of acquisition, mechanisms of transmission, and spread; identify risk factors associated with infection and disease; characterize and relate causal factors to an infectious disease; determine or select appropriate methods of prevention and control; or guide rational application and practice of clinical microbiology methods. These epidemiologic methods were developed in an attempt to control common errors in observations that occur when one studies the association of one event (a risk or causal factor) with another later event (the outcome or disease).
or regions. Such methods are used to determine the exposure-disease relationship in humans; establish the modes of acquisition, mechanisms of transmission, and spread; identify risk factors associated with infection and disease; characterize and relate causal factors to an infectious disease; determine or select appropriate methods of prevention and control; or guide rational application and practice of clinical microbiology methods. These epidemiologic methods were developed in an attempt to control common errors in observations that occur when one studies the association of one event (a risk or causal factor) with another later event (the outcome or disease).
Epidemiologic study methods are grouped as either observational or experimental. Observational epidemiologic methods are further classified as either descriptive or analytic. Observational studies are conducted in natural, everyday community or clinical settings, where the investigators observe the appearance of an outcome but have no control over the environment or the exposure of people or product to a risk factor or suspected etiologic agent, a specific intervention or preventive measure, or a particular therapeutic regimen.
Descriptive Epidemiology
Observational descriptive studies establish the case definition of an infectious disease event by obtaining data for analysis from available primary (e.g., medical records) or secondary (e.g., infection control surveillance) sources. These data enable the characteristics of the population that has acquired the infection to be delineated according to (a) “person” (age, sex, race, marital status, personal habits, occupation, socioeconomic status, medical or surgical procedure or therapy, device use, underlying disease, or other exposures or events); (b) “place” (geographic occurrence of the health event or outbreak, medical or surgical service, place of acquisition of infection, or travel); and (c) “time” (preepidemic and postepidemic periods, seasonal variation, secular trends, or duration of stay in hospital). The information from descriptive studies might provide important clues regarding the risk factors associated with infection, and in each case it is hoped that an analysis of the collected data might be used to generate hypotheses regarding the occurrence and distribution of disease or infection in the population(s) being studied.
Analytic Epidemiology
Observational analytic studies are designed to test hypotheses raised by the findings in descriptive investigations. The objectives of these studies are (a) to establish the cause and effects of infection in a population and (b) determine why a population acquired a particular infection in the first place. The three most common types of observational analytic studies are cohort studies, case-control studies, and prevalence or cross-sectional studies.
Cohort Studies In cohort studies, hypotheses that have been generated from previous (descriptive) studies are tested in a new population. A population of individuals (a cohort) that is free of the infection or disease of interest is recruited for study. The presence or absence of the suspected (hypothesized) risk factors for the disease is recorded at the beginning of the study and throughout the observation period. All members of the cohort population (e.g., all premature infants admitted to a neonatal intensive care unit during a defined time period) are followed over time for evidence or appearance of the infection or disease and classified accordingly as exposed or unexposed to specific risk factors. If the observation period begins at the present time and continues into the future or until the appearance of disease, the study is called a prospective cohort study. If the population studied is one that in the past was apparently free of the markers of disease on examination of records or banked laboratory specimens, it may be chosen for study if data on exposure to the suspected risk factors for disease also are available. The population may be followed to the present or until the appearance of disease. This type of study, common in occupational epidemiology, is called a historical or retrospective cohort study.
A key requirement of a cohort study is that participants be reliably categorized into exposed and unexposed groups. Relative risk, that is, the ratio of the incidence of the outcome in the exposed group to the incidence in the unexposed group, is used to measure the strength of an association between exposures or risk factors and disease. Cohort studies have the advantage of enabling identification and direct measurement of risk factors associated with disease, determination of the incidence of infection and disease, and ascertainment of the temporal relationship between exposure and disease. In cohort studies, observational bias may be less of a limitation on the validity or results, since the information on the presence of risk factors is recorded before the outcome of disease is established. To ensure sufficient numbers for analysis, cohort studies require continual follow-up of large populations for long periods unless the disease under investigation is one of high incidence. Cohort studies are, in general, more expensive and time-consuming to conduct and are not suitable for the investigation of uncommon infections or conditions. However, they render the most convincing nonexperimental approach for establishing causation.
Case-Control Studies In a case-control study, individuals (cases) who are already infected, ill, or meet a given case definition are compared with a group of individuals (controls) who do not have the infection, disease, or other outcome of medical interest. In contrast to cohort studies, participants in a case-control study are selected by manifestation of symptoms and signs, laboratory parameters, or a specific condition, disease, or outcome. Thus, the search for exposure of case and control subjects to potential risk factors remains a retrospective one. For case-control studies, the measure of association between exposures or risk factors and health outcome is expressed as an odds ratio, that is, the ratio of the odds of an exposure, event, or outcome occurring in a population to the odds in a control group, where the odds of an event is the ratio of the probability of it occurring to the probability of it not occurring.
The presence of significant differences in the exposure to risk factors among case versus control subjects suggests an etiologic (causal) association between those factors and the infection or disease defined by cases. Casecontrol methods are useful for studying infections, events, or outcomes likely associated with multiple risk factors or
low incidence rates; for investigating situations in which there is a long lag-time between exposure and outcome of interest; and for establishing etiologic associations or causation of a disease, infection, or other outcome when there is no existing information about the cause or source. In an attempt to reduce bias, control subjects might be selected from individuals matched with cases for selected characteristics, such as age, gender, socioeconomic status, or other variables not suspected or under investigation as risk factors. Compared with cohort studies, case-control studies may be conducted in relatively shorter time, are relatively less expensive, or may require a smaller sample size to execute. Limitations of case-control studies include selection bias in choosing case and control subjects; recall bias in which study subjects might have difficulty in remembering possible exposures; incomplete information on specific exposures; or risk factor data may be difficult to find (or remember). Case-control studies are not used to measure incidence or prevalence rates and, generally, are not capable of establishing temporal relationships between an exposure and outcome.
low incidence rates; for investigating situations in which there is a long lag-time between exposure and outcome of interest; and for establishing etiologic associations or causation of a disease, infection, or other outcome when there is no existing information about the cause or source. In an attempt to reduce bias, control subjects might be selected from individuals matched with cases for selected characteristics, such as age, gender, socioeconomic status, or other variables not suspected or under investigation as risk factors. Compared with cohort studies, case-control studies may be conducted in relatively shorter time, are relatively less expensive, or may require a smaller sample size to execute. Limitations of case-control studies include selection bias in choosing case and control subjects; recall bias in which study subjects might have difficulty in remembering possible exposures; incomplete information on specific exposures; or risk factor data may be difficult to find (or remember). Case-control studies are not used to measure incidence or prevalence rates and, generally, are not capable of establishing temporal relationships between an exposure and outcome.
Prevalence or Cross-Sectional Studies In prevalence studies, the presence of putative risk factors and the disease under investigation is recorded in a survey of a study population at a specific point in time or within a (short) time period. The rates of disease among those with and without the suspected risk factors are compared. Thus, cross-sectional studies can establish association but not causation for suspected risk factors. Prevalence studies are relatively inexpensive and can be carried out rapidly if well-planned. However, they do not allow the ascertainment of risk factors at the beginning of disease nor do they enable one to establish a temporal sequence of risk factors preceding the infection or other outcome of interest. Point prevalence, period prevalence, and seroprevalence surveys are examples of cross-sectional studies.
Experimental Epidemiology
In experimental studies, the investigator controls an exposure of individuals in a population to a suspected causal factor, a prevention measure, a therapeutic regimen, or some other specific intervention. These exposure modalities are randomly allocated to comparable groups, thereby minimizing confounding factors. Both the exposed and unexposed groups are monitored thereafter for specific outcomes (e.g., appearance of infection or disease, evidence of effective prevention or control of the disease, or cure). Experimental studies often are used to evaluate antimicrobial or vaccine treatment regimens and are generally expensive to conduct. Within healthcare settings, studies that examine restriction of certain antimicrobials or promotion of use of alternative antimicrobials for the control of antimicrobial resistance could be considered under the category of experimental. For ethical reasons, it is rarely possible to expose human populations to potential pathogens or to withhold a preventive measure that could potentially be beneficial to the patient. Unfortunately, animal hosts are not naturally susceptible to many agents of human disease. Thus, one has to be careful when extrapolating epidemiologic findings in animal experimental studies to the control of infections in human subjects.
Quasi-experimental studies: more recently, there has been an increase in the number of published papers describing results from these studies. This type of study shares the design characteristics of experimental studies but lacks random assignments of study subjects. Quasi-experimental studies are useful where randomization is impossible, impractical, or unethical. The main drawbacks of quasiexperimental studies are their inability to eliminate confounding bias or establish causal relationships.
EPIDEMIOLOGY OF INFECTION AND DISEASE
The epidemiology of infectious disease presents two processes for discussion: (a) the epidemiology of the determinants leading to infections in hosts and (b) the epidemiology of the appearance and extent of disease related to the infection in those hosts. It is common to discuss health and disease as the result of a series of complex interactions between an agent of change, the host that is the target of the agent’s actions, and the mutual environment in which the host and agent are found. In studies of healthcare-associated infections, the agents are the microorganisms associated with the infections, the hosts are the patients under care or their healthcare workers, and the common environment is the acute care hospital, intensive care unit, outpatient, home, or other healthcare venues.
The interactions determining the probability of a microbiologic agent causing infection in a host may be simply presented by an equation of infection:
Ip = (D × S × T × V)/Hd,
where Ip is the probability of infection, D is the dose (number of microorganisms) transmitted to the host, S is the receptive host site of contact with the agent, T is the time of contact (sufficient for attachment and multiplication or not), and V represents virulence, the intrinsic characteristics of the microorganism that allow it to infect. The denominator in the equation (Hd) represents the force of the combined host defenses attempting to prevent this infection.
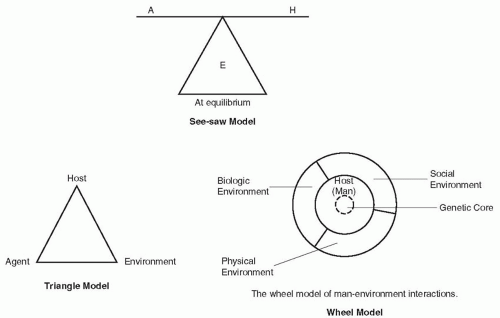
Any reduction in host defenses (represented by the denominator) in such an equation allows infection to take place with a similar reduction in one or more of the agent factors in the numerator. Infection may take place with a smaller dose of microorganisms. Infection may take place at an unusual site. The contact time for a microorganism to fix to an appropriate surface may be briefer, or infection may take place with an agent of lesser virulence, one that does not cause infection in the normal host. These reductions in the host defense characteristics, represented by the denominator, and the reduction of requirements to infect for the agent are typical of the interactions that allow opportunistic infections in compromised hosts, represented by many patients under care in modern hospitals. In this model, equation of infection, the environment might be considered the background or playing field on which the agent-host interaction takes place. A number of additional models of the interaction of agent, host, and environment have been suggested to help understand these processes. The three models in Figure 1-1—the seesaw model, the
triangle model, and the wheel model—have been frequently cited (9,10). Each attempts to simply visualize the interplay between the three components.
triangle model, and the wheel model—have been frequently cited (9,10). Each attempts to simply visualize the interplay between the three components.
INTERACTIONS OF AGENT, HOST, AND ENVIRONMENT
All outcome events (infection or disease) have multifactorial causes. For some infectious diseases, a single unique factor or agent is necessary and sufficient for the disease to appear. This is exemplified by measles or rabies. It is only necessary for the host to be exposed to and infected by an agent (the measles virus or the rabies virus) for that disease to develop. For other infectious diseases, the single factor of infectivity of the agent is necessary but not sufficient to cause disease in the host. M. tuberculosis, polio virus, hepatitis A, and many other agents necessary for specific disease in a human host infect without causing disease in a majority of cases. Within the hospital setting, exposure to a specific microorganism or colonization of an inpatient with an agent, such as vancomycin-resistant enterococcus (VRE) or Staphylococcus aureus, may be necessary but not sufficient to generate disease, which only develops through complex interactions between other contributory factors, such as age, state of debilitation, immune or nutritional status, device use, invasive procedures, antimicrobial usage, or susceptibility of the microorganism to available antimicrobials. The fact of the infection in these cases is not sufficient to produce disease in the host without the contribution of these latter elements in the host and the environment.
Agent
The agents causing healthcare-associated infectious diseases are microorganisms ranging in size and complexity from viruses and bacteria to protozoa and helminths. Bacteria, fungi, and certain viruses have been the agents most recognized and studied as causes of healthcare-associated infections (11). For transmission to take place, the microorganism must remain viable in the environment until contact with the host has been sufficient to allow infection. Reservoirs that allow the agent to survive or multiply may be animate, as exemplified by healthcare worker carriage of staphylococci in the anterior nares or throat (12,13, 14, 15), or the inanimate environment, as demonstrated by Pseudomonas spp. colonization of sink areas, Legionella in hot or cold water supply systems (16, 17, 18, 19), Clostridium difficile spores on computer keyboards, or Serratia marcescens growing in contaminated soap or hand lotion preparations (20, 21, 22).
Certain intrinsic and genetically determined properties of a microorganism are important for it to survive in the environment. These include the ability to resist the effects of heat, drying, ultraviolet light, or chemical agents, including antimicrobials; the ability to compete with other microorganisms; and the ability to independently multiply in the environment or to develop and multiply within another host or vector. Intrinsic agent factors important to the production of disease include infectivity, pathogenicity, virulence, the infecting dose, the agent’s ability to produce toxins, its immunogenicity and ability to resist or overcome the human immune defense system, its ability to replicate only in certain types of cells, tissues, or hosts (vectors), its
ability to persist or cause chronic infection, and its interaction with other host mechanisms, including the ability to cause immunosuppression (e.g., HIV).
ability to persist or cause chronic infection, and its interaction with other host mechanisms, including the ability to cause immunosuppression (e.g., HIV).
Once transferred to a host surface, the agent may multiply and colonize without invading or evoking a measurable host immune response (23, 24, 25). The presence of an agent at surface sites in the host does not define the presence of an infection. Nonetheless, patients so colonized may act as the reservoir source of transmission to other patients (26).
If infection takes place, a measurable immune response will develop in most hosts even if the infection is subclinical. The success of this process for the agent is increased in the nonimmune host and is most successful in the nonimmune, immunocompromised host. A microorganism’s ability to infect another host vector (e.g., yellow fever virus in mosquitoes) or another nonhuman reservoir (e.g., yellow fever virus in the monkey) is important in the epidemiology of certain infectious diseases in world populations at large but plays little role in healthcare infection epidemiology.
Host
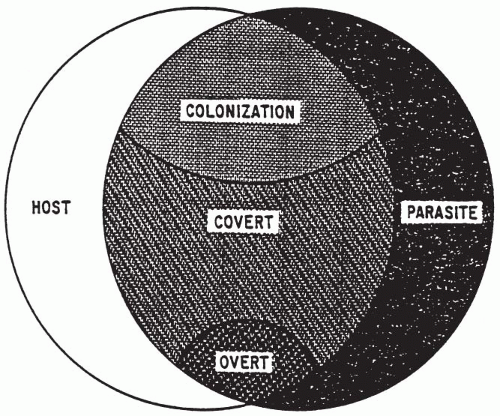
Infection depends on exposure of a susceptible host to an infecting agent. Exposure of the susceptible host to such agents is influenced by age, behavior, family associations, occupation, socioeconomic level, travel, avocation, access to preventive healthcare, vaccination status, or hospitalization. Whether or not disease takes place in the infected host and the severity of disease when it appears depend not only on the intrinsic virulence factors of the agent but more importantly on the pathogenicity of the interactions between the agent and the host. The host immune defenses attempt to prevent infection. Thus, any reduction in host defenses may allow infection to take place with a smaller dose of microorganisms or at a body site that is not usually susceptible to infection. A combination of reductions in host defense characteristics and the requirements for an agent to cause infection are typical of the interactions that allow acquisition of opportunistic infections in immunocompromised patients. A commonly cited model indicating the potential interactions between agent and host and the relationships among colonization, infection, and clinical and subclinical disease is shown in Figure 1-2 (27).
Host factors important to the development and severity of infection or disease may be categorized as intrinsic or extrinsic. Intrinsic factors include the age at infection; birth weight; sex; race; nutritional status (28); comorbid conditions (including anatomic anomalies) and diseases; genetically determined immune status; immunosuppression associated with other infections, diseases, or therapy; vaccination or immunization status; previous experience with this or similar agents; and the psychological state of the host (29). Colonization of the upper and lower respiratory tracts is more likely when the severity of illness increases in critically ill patients. This, along with other host impairments (e.g., reduced mucociliary clearance or changes in systemic pH), allows colonization to progress to invasive infection. Moreover, other clinical conditions may lead to an alteration in epithelial cell surface susceptibility to binding with bacteria, leading to enhanced colonization (23, 24, 25). Extrinsic factors include invasive medical or surgical procedures; medical devices, such as intravenous catheters or mechanical ventilators; sexual practices and contraception; duration of antimicrobial therapy and hospitalization; and exposure to hospital personnel.
Environment
The environment provides the mutual background on which agent-host interactions take place and contains the factors that influence the spread of infection. Environmental factors include (a) physical factors such as climatic conditions of heat, cold, humidity, seasons, and surroundings (e.g., intensive care units, outpatient clinics, long-term care facilities, or water reservoirs); (b) biologic factors (e.g., intermediary hosts such as insect or snail vectors); and (c) social factors (e.g., socioeconomic status, sexual behavior, types of food and methods of preparation, and availability of adequate housing, potable water, adequate waste disposal and healthcare amenities). These environmental factors influence both the survival and the multiplication of infectious disease agents in their reservoirs and the behavior of the host in housing, occupation, and recreation that relate to exposure to pathogens. Food- and water-borne diseases flourish in warmer months because of better incubation temperatures for the multiplication of the agent and recreational exposures of the host, whereas respiratory agents appear to benefit from increased opportunities for airborne and droplet transmission in the closed and closer living environments of the winter. In US hospitals, the frequency of hospital-acquired Acinetobacter spp. infections is increasing in critical care units and has been shown to be seasonal in nature (30). The seasonal variation in the incidence of this pathogen is thought to be due to changes in climate—summer weather increases the number of Acinetobacter spp. in the natural environment and transmission of this microorganism in the hospital environment during this season (30).
Within healthcare settings, the components of the agent, host, and environment triad interact in a variety of ways to produce healthcare-associated infections. For example, the
intensive care unit is now considered the area of highest risk for the transmission of healthcare-associated pathogens in US hospitals (31). Moreover, methicillin-resistant S. aureus (MRSA), VRE, and ceftazidime-resistant Pseudomonas aeruginosa are endemic in many intensive care units in these hospitals (31). The emergence of vancomycin-resistant S. aureus in US institutions highlighted the unwelcome but inevitable reality that this pathogen may become endemic in acute care settings (32). A complex interaction of contributory factors, such as inadequate hand washing and infection control practices among healthcare workers, fluctuating staffing levels, an unexpected increase in patient census relative to staffing levels in the intensive care unit, or an unprecedented increase in the number of severely ill patients with multiple invasive devices, could all contribute to the acquisition of hospital infections caused by one of these endemic microorganisms (33,34). Adding to the complexity of the process would be the unquantifiable mechanism of transmission of the agent from host to healthcare worker, healthcare worker to healthcare worker, and host to environment. Thus, acceptable measures for the prevention and control of healthcare-associated infection dictate that the healthcare epidemiologist looks at and analyzes the interrelationships among all components of the triad of agent, host, and environment (31).
intensive care unit is now considered the area of highest risk for the transmission of healthcare-associated pathogens in US hospitals (31). Moreover, methicillin-resistant S. aureus (MRSA), VRE, and ceftazidime-resistant Pseudomonas aeruginosa are endemic in many intensive care units in these hospitals (31). The emergence of vancomycin-resistant S. aureus in US institutions highlighted the unwelcome but inevitable reality that this pathogen may become endemic in acute care settings (32). A complex interaction of contributory factors, such as inadequate hand washing and infection control practices among healthcare workers, fluctuating staffing levels, an unexpected increase in patient census relative to staffing levels in the intensive care unit, or an unprecedented increase in the number of severely ill patients with multiple invasive devices, could all contribute to the acquisition of hospital infections caused by one of these endemic microorganisms (33,34). Adding to the complexity of the process would be the unquantifiable mechanism of transmission of the agent from host to healthcare worker, healthcare worker to healthcare worker, and host to environment. Thus, acceptable measures for the prevention and control of healthcare-associated infection dictate that the healthcare epidemiologist looks at and analyzes the interrelationships among all components of the triad of agent, host, and environment (31).
It is well-known that the social environment is extremely significant in determining personal behavior that affects the direct transmission of agents, such as HIV via breast milk in regions of high HIV endemicity, gram-negative microorganisms via artificial nails worn by healthcare workers in US intensive care units (35), and pathogens that cause sexually transmitted diseases. What must be understood to be equally relevant is the impact of other factors in the social environment, such as the distribution of and access to medical resources; the use of preventive services (36, 37, 38); the enforcement of codes in food preparation, infection control practices, or occupational health practices; the extent of acceptance of breast-feeding for children (39, 40, 41); and the acceptance of advice on the appropriate use of antimicrobials (42, 43, 44,45,46). Also, there must be an appreciation by patients, relatives, and healthcare workers alike that at-risk patients (e.g., those born very prematurely have severe congenital abnormalities, the very elderly, or those with premorbid end-stage cardiac or pulmonary disease), who have numerous indwelling medical invasive devices, or who have undergone multiple invasive procedures or surgical procedures would be particularly susceptible to healthcare-associated infections that are likely nonpreventable. There must be an informed and ethically sound willingness to reject the extraordinary application of medical technology, including the inappropriate or repeated use of resistance-inducing antimicrobials when clinical evidence and experience suggest that the condition of the sick patient is untreatable or irreversible.
Special Environments
Microenvironments, including military barracks, dormitories, day-care centers, chronic disease institutions, ambulatory surgery and dialysis centers, and acute care hospitals, provide special venues for agent-host interactions. Historically, epidemics in these institutional environments provided the experience that drove the development and acceptance of control measures, guidelines, and infection control programs. Acute care hospitals, especially those offering regional secondary and tertiary care, remain the dominant examples of these environments. Changing patterns of outpatient practice, home healthcare, and technical advances in medicine have resulted in increasingly severely diseased and injured populations being managed in acute care facilities. Data from CDC demonstrate that the changing healthcare environments in the United States are resulting in larger intensive care unit populations while there has been a general decrease in the number of general medical beds (31).
Special units for intensive medical or surgical care for extensive burns, trauma, transplantation, and cancer chemotherapy frequently house patients with increased susceptibility to infection (47). In these patients, reduced inocula of pathogens or commensals are required to cause infection, infection may take place at unusual sites, and usually nonpathogenic agents may cause serious disease and death. Frequent opportunistic infections in these patients require repeated, broad, and extended therapy with multiple antimicrobials, leading to increasingly resistant resident microbial populations (31,46).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree