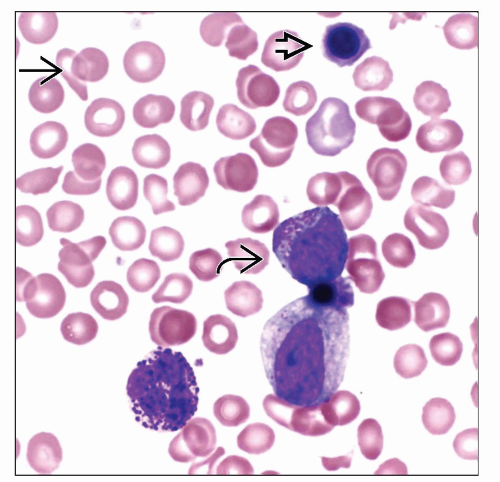
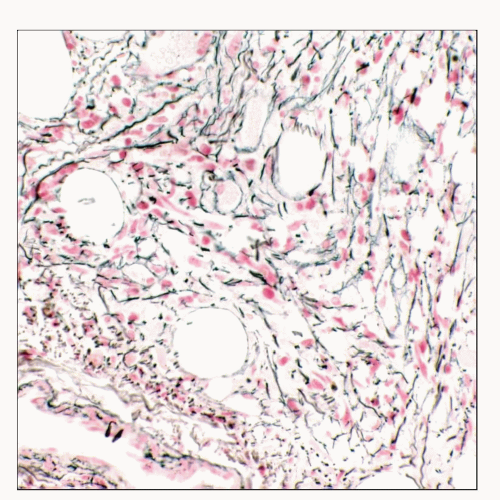
Polycythemia Vera
Mohammad A. Vasef, MD
Carmen Frias M. Kletecka, MD
Key Facts
Terminology
Subtype of classic chronic myeloproliferative neoplasm characterized in 2008 WHO by
Increased red blood cells
JAK2 gene gain-of-function somatic mutation
3 polycythemia vera phases
Prepolycythemic phase with mild erythrocytosis
Overt polycythemic phase with significant increased red blood cell mass
Spent phase and postpolycythemic myelofibrosis
Clinical Issues
Laboratory tests useful in work-up of suspected PV
Serum erythropoietin (EPO) value
Endogenous erythroid colony formation
Tests for JAK2 V617F or JAK2 exon 12 mutations
Prognosis
Median survival > 10 years with treatment
High risk with history of thrombosis and age > 60
Progression to MDS or AML in 2-10% of cases
Ancillary Tests
Common recurring cytogenetic abnormalities
Trisomy 8, trisomy 9, del(20q), del(13q), and del(9p)
JAK2 V617F mutation
Present in 96% of patients with PV
JAK2 exon 12 mutations
3% frequency among all patients with PV
Top Differential Diagnoses
Chronic myeloid leukemia (CML)
Essential thrombocythemia (ET)
Primary myelofibrosis (PMF)
TERMINOLOGY
Abbreviations
Polycythemia vera (PV)
Synonyms
Polycythemia rubra vera
Definitions
Classic chronic myeloproliferative neoplasm (MPN) characterized in 2008 WHO by
Increased red blood cells
Janus kinase 2 (JAK2) gene gain-of-function somatic mutation
3 phases of polycythemia vera
Prepolycythemic phase with mild erythrocytosis
Overt polycythemic phase with significantly increased red blood cell (RBC) mass
Spent phase and postpolycythemic myelofibrosis
Normalization followed by decrease in RBC mass
Further enlargement of spleen
Marked reticulin and collagen fibrosis of marrow
Extramedullary hematopoiesis (EMH)
ETIOLOGY/PATHOGENESIS
Underlying Etiology of PV
Genetic disposition reported in some families
Ionizing radiation and occupational toxin exposure suggested as possible cause
Underlying cause is uncertain in most patients
CLINICAL ISSUES
Epidemiology
Incidence
Estimated 2-3 per 100,000 persons affected each year
Higher incidence among Ashkenazi Jews
Very low incidence in Japan
Slight male predominance
Average age at diagnosis is 60 years
Rare in patients < 30 years
Presentation
Patients frequently present with thrombotic events
Nonspecific symptoms such as headaches, dizziness, pruritus, and visual disturbances may exist
Weight loss and arthropathies due to gout may be seen
Hepatosplenomegaly, ruddy cyanosis, conjunctival plethora, and hypertension can be detected on exam
Laboratory Tests
Laboratory tests useful in work-up of suspected PV
Serum erythropoietin (EPO) value
Endogenous erythroid colony formation
Tests for JAK2 V617F or JAK2 exon 12 mutations
Treatment
PV is not curative by current drug therapy
Low-dose aspirin plus phlebotomy for management of low-risk PV patients
Low-dose aspirin plus phlebotomy plus hydroxyurea in high-risk PV patients
Allogeneic stem cell transplantation (SCT) can be potentially curative in post-PV myelofibrosis
Allogeneic SCT has limited usage due to high incidence of mortality and morbidity
Investigational drug therapies in post-PV myelofibrosis
JAK2 inhibitor (TG101348) in phase I/II study
≥ 50% reduction in spleen size during the 1st 6 months of therapy in about 50% of cases
Normalization of leukocytosis and thrombocytosis in most patients
≥ 50% reduction in JAK2 V617F mutated allele burden in some patients
JAK1 & JAK2 inhibitor (INCB018424), phase I/II
Decrease in spleen size in > 40% of patients
Effective control of erythrocytosis in PV patients
Normalization of thrombocytosis and decreased leukocytosis in > 50% of cases with leukocytosis
Subset of transfusion-dependent patients became transfusion independent
No significant effect on JAK2 V617F allele burden
Prognosis
Median survival: > 10 years with treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree