Pituitary
SURGICAL CLINICAL CONSIDERATIONS
Goal of Consultation
Ensure diagnostic tissue has been obtained
Render diagnosis when possible
Identify microadenomas
Ensure completeness of resection
Change in Patient Management
Additional tissue will be biopsied until diagnostic tissue is obtained
Lesions causing symptoms due to mass effect will be completely excised, if possible
Specific diagnosis can help guide surgery
Functional tumors
Pituitary adenomas (including microadenomas), pituitary hyperplasia
Diagnosed prior to surgery due to symptoms and elevated serum levels of hormones
Adenoma cannot be distinguished from hyperplasia preoperatively
Diagnosis can be confirmed intraoperatively
If tumor is not completely resected, radiation therapy may be required for treatment
Tumors with high risk of recurrence
Rathke cleft cyst, spindle cell oncocytoma, meningioma, craniopharyngioma, invasive pituitary adenoma
May require resection of adjacent structures to prevent recurrence
Absence of cavernous sinus invasion may stop surgery for invasive pituitary adenomas
Tumors with low risk of recurrence
Epidermoid and dermoid cysts, paraganglioma, gangliocytoma
Removal of main tumor mass is often sufficient for cure
Lesions not requiring surgical resection
Plasmacytoma, lymphoma
Treated systemically
Specimens are small, and frozen section artifact can preclude optimal evaluation
Tissue should only be frozen if diagnosis will alter surgical procedure or if surgeon encounters an unexpected finding
Clinical Setting
Patients with pituitary tumors usually present with symptoms due to abnormal function, compression of adjacent structures, or both
Overproduction of hormones
Adrenocorticotrophic hormone (ACTH): Causes adrenal glands to make cortisol (Cushing syndrome)
Fat accumulation and upper back hump
Hypertension
Thin skin and striae
Hyperglycemia
Anxiety, irritability, or depression
Growth hormone
Gigantism: Accelerated and excessive growth in children and adolescents
Acromegaly: Coarsened facial features and enlarged hands and feet in adults
Hyperglycemia
Prolactin
Can be caused by an adenoma or any lesion obstructing the pituitary stalk (blood flow normally resulting in inhibition is diminished)
Decrease in sex hormones
Women: Galactorrhea, irregular or absent menstrual periods
Men: Loss of sex drive, infertility
Thyroid-stimulating hormone
Hyperthyroidism: Tachycardia, weight loss, hyperthermia
Compression of adjacent structures &/or pituitary
Headache, nausea, and vomiting
Vision loss, especially peripheral vision
Pituitary hormone deficiency
SPECIMEN EVALUATION
Gross
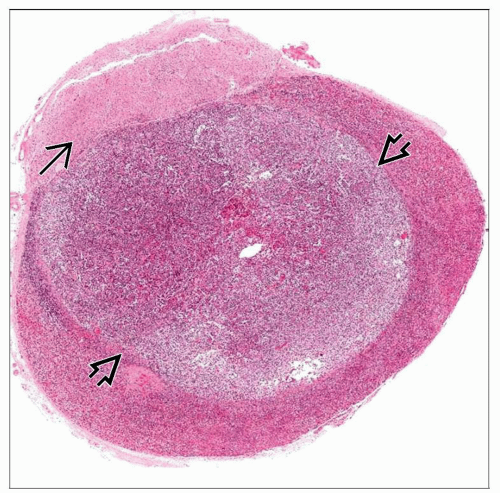
Pituitary lesions are typically resected as multiple small fragments
Adenomas often have a soft white, creamy appearance
Specimen does not need to be inked
Document size, number of fragments, and color
Frozen Section
Tissue can be completely frozen after making cytologic preparations
Frozen section is best technique to demonstrate architectural pattern
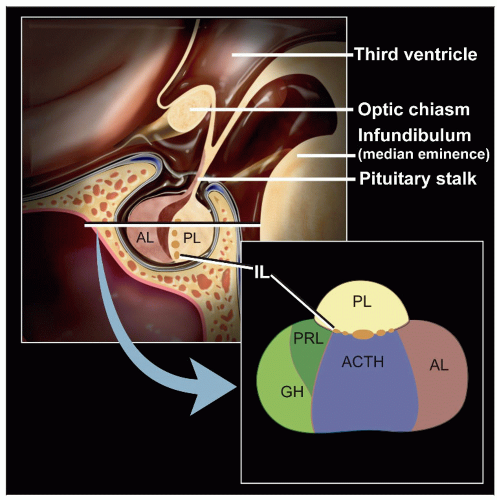
Lobular pattern of normal gland
Diffuse lobular expansion of adenoma
Nuclei of adenomas can appear to be pleomorphic due to frozen section artifact
If sufficient tissue is present, nonfrozen tissue should be saved for permanent sections and possible ancillary studies
Cytology
Cytologic preparations (smear or touch preparations) are very helpful to evaluate pituitary adenomas
Delicate, loosely cohesive cells
Typical neuroendocrine (“salt and pepper”) chromatin pattern readily appreciated
Monomorphic population
Squash preparations can lead to disintegration of cytoplasm and naked nuclei due to delicate nature of adenomas
MOST COMMON DIAGNOSES
Pituitary Hyperplasia
Occurs secondary to hypersecretion of stimulating hormone by either physiologic or pathologic mechanisms
Most common cause is hypothyroidism
Gland is enlarged and lacks a well-defined mass distinguishable from surrounding normal gland
May be diffuse within gland or focal with formation of nodules
Anterior gland is composed of enlarged acini, composed mostly of a single cell type
Focal or diffuse expansion of acini with preservation of reticulin pattern (reticulin stain)
Pituitary Adenoma
90% of lesions of sellar region are pituitary adenomas
Symptoms often due to overproduction of pituitary hormones
Diagnosis is usually known prior to surgery
Classification as to type of adenoma is not important for intraoperative consultation
Architectural features
Diffuse lobular expansion
Normal glands are organized in lobules
Epithelial cords and sheets with dyscohesive ends, acini, and papillary formations are seen on cytology
Solid, diffuse, trabecular, sinusoidal, and papillary growth patterns are common
Perivascular pseudorosette formation and solid papillary growth patterns are usually seen in gonadotroph adenomas
Cystic changes can occur
Lack of calcifications distinguish cystic adenomas from craniopharyngioma
Nuclear features
Typical neuroendocrine appearance (finely dispersed chromatin with distinct nucleoli)
Mild cellular pleomorphism and binucleate forms are common
Monomorphic appearance
Cytoplasmic features
Cytoplasmic granularity and staining identifies 3 morphologically distinct cell types
Eosinophilic: Characteristic of acidophilic adenomas that produce growth hormone (GH) (somatotroph adenomas) or prolactin (lactotroph adenomas), but can be nonfunctional
Basophilic: These adenomas produce adrenocorticotrophic hormone (ACTH) (corticotrophic adenomas), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) (gonadotrophic adenomas) or thyroid-stimulating hormone (TSH) (thyrotropic adenomas), but can be nonfunctional
Chromophobic: These adenomas are usually nonfunctional but may produce TSH
Normal pituitary glands are composed of all 3 cell types: Eosinophilic, basophilic, and chromophobic
Adenomas are composed of a single cell population
Cytoplasmic contents, depending on functional status, are variably clear, with vacuolation or eosinophilic bodies and occasional paranuclear bodies
Necrosis and mitoses are uncommon
Psammoma bodies may be present
Most common in adenomas producing TSH or prolactin
Invasive adenomas
Local extension can involve bone, posterior lobe, dura mater, or respiratory mucosa
Not an indication of malignancy or capacity for metastasis
May recur locally
Cytologic features do not predict invasiveness
Microadenoma
< 1 cm in size
May be functional or nonfunctional (“incidentaloma”)
Crooke cell adenoma
Corticotroph (producing ACTH) adenoma
Cytokeratin accumulates in cytoplasm in response to increased glucocorticoids (Crooke hyaline change)
3/4 are invasive and > 1/2 recur locally
Pituitary Carcinoma
No combination of histologic features diagnostic of carcinoma
Presence of invasion, cellular pleomorphism, mitosis, or necrosis are not sufficient for diagnosis of malignancy
Diagnosis of pituitary carcinoma is dependent upon demonstration of metastases
Mitotic activity is increased in carcinomas (up to 67%), but there is considerable overlap with adenomas
Posterior Pituitary Lesions
Anatomically consists of pars nervosa and infundibular stalk
Composed of axons of magnocellular neurosecretory cells
Axons store and release oxytocin and vasopressin
Pituicytes are specialized glial cells resembling astrocytes that function in storage and release of hormones
Symptoms can be due to loss of pituitary function, compression of adjacent structures (e.g., visual disturbance), or (less commonly) increase in pituitary hormones
Lesions of this region include
Granular cell tumor
Hypothalamic hamartoma
Sarcoidosis
Presents with hypopituitarism
Almost all patients have systemic sarcoidosis
Only 1% have lesions limited to central nervous system
Langerhans cell histiocytosis
Pituicytoma
Low-grade glioma
Mitoses rare
Hypothalamic or optic pathway astrocytomas
Craniopharyngioma
Benign slow-growing suprasellar solid and cystic tumor arising from embryologic remnants of Rathke pouch
Growth due to an increase in tumor cells
Symptoms of headache, pituitary dysfunction, and visual disturbances are due to impingement on adjacent structures
Leakage of contents can cause aseptic meningitis
May recur if entire tumor is not removed
Can be adherent to adjacent structures
Cytologic features are “wet” keratin and clusters of squamous cells with a palisaded border
2 histological variants
Adamantinomatous craniopharyngioma
Usually in children but also adults
Composed of cords or islands of epithelial cells in loose fibrous stroma with intervening cysts
Resemble adamantinoma (most common type of tooth tumor)
Outer layer of epithelium is palisaded
“Wet” keratin associated with nuclear dropout to form ghost keratinocytes
Cholesterol clefts, desquamated keratin, calcification
Calcifications can be seen on imaging and are helpful for diagnosis
Occasional inflammatory component and gliosis
Papillary craniopharyngioma
Almost all in adults
Stratified squamous epithelium with papillary projections
Solid growth pattern
Usually has a smooth outer surface and is not adherent to adjacent structures
Lacks palisading, fibrosis, “wet” keratinization, and calcifications
Rathke Cleft Cyst
Benign fluid-filled cyst formed if Rathke pouch does not develop normally
Symptoms occur due to compression of adjacent structures
Growth occurs due to fluid accumulation
Visual disturbances are most common, followed by pituitary dysfunction
Lined by columnar cells with cilia and goblet cells
Cytology shows scattered clusters of cuboidal cells with prominent cilia
Presence of extensive squamous change can mimic a craniopharyngioma
Hypocellular cystic craniopharyngiomas can be mistaken for epidermoid or Rathke cleft cysts
Epidermoid Cysts
Arise from embryological remnants
Growth due to accumulation of keratin debris
Usually present in young adults (20-40 years of age)
Symptoms due to compression of adjacent structures
Unilocular cysts lined by orderly stratified squamous epithelium
Calcification is rare in epidermoid cysts
Neuronal Cell Tumors
Relatively rare lesions
Neuronal tumors of the sellar region include paraganglioma, gangliocytoma, glioma, spindle cell oncocytoma, granular cell tumor, pituicytoma, schwannoma, meningioma, and neuroblastoma
Inflammatory Hypophysitis
Primary inflammatory hypophysitis
Rare disorder characterized by focal or diffuse inflammatory infiltration and ultimate destruction of pituitary gland
Thought to be an autoimmune disease localized to pituitary
Can affect anterior lobe, posterior lobe, or both
Symptoms depend on areas of pituitary with loss of function
Diagnosis requires biopsy
Classified into 3 histopathological categories
Lymphocytic hypophysitis
Infiltration of anterior pituitary by lymphocytes and plasma cells with occasional germinal centers
Parenchymal atrophy, variable degree of fibrosis, and residual lymphocytic infiltration at later stages of disease
Granulomatous hypophysitis
Well-formed, noncaseating granulomas associated with variable lymphocytic infiltrates
Xanthomatous hypophysitis
Variable lymphoplasmacytic inflammatory infiltrates
Foamy macrophages with giant cell formation, necrosis, and hemosiderin deposition
Secondary inflammatory hypophysitis
Gangliocytoma
May present with acromegaly or mass effect symptoms
Composed of large mature ganglion cells with abundant cytoplasm
Proliferative glial stroma with Rosenthal fibers
Metastatic Carcinoma
Secondary tumors of pituitary gland result from either hematogenous spread or direct invasion
Tumor cells are large, with nuclear pleomorphism, and some with glandular arrangements
Involvement of posterior lobe is more common
Portal nature of vascularization of adenohypophysis has been thought to form a protective barrier
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree