Personal Protective Equipment
Joshua G. Schier
Robert S. Hoffman
INTRODUCTION
A hazardous substance (HS) may be defined simply as any substance that is potentially toxic to living systems (1). A more technical definition of a HS, as defined by the U.S. Occupational Safety and Health Administration (OSHA), is any substance designated or listed under (A) through (D) of this definition, exposure to which results or may result in adverse effects on the health or safety of employees:
[A] Any substance defined under section 101 (14) of CERCLA (Comprehensive Environmental Response, Compensation and Liability Act or CERCLA, also known as Superfund); [B] Any biologic agent and other disease causing agent which after release into the environment and upon exposure, ingestion, inhalation, or assimilation into any person, either directly from the environment or indirectly by ingestion through food chains, will or may reasonably be anticipated to cause death, disease, behavioral abnormalities, cancer, genetic mutation, physiological malfunctions (including malfunctions in reproduction) or physical deformations in such persons or their offspring. [C] Any substance listed by the U.S. Department of Transportation as hazardous materials under 49 CFR 172.101 and appendices; and [D] Hazardous waste (2). Hazardous waste is defined as: [A] A waste or combination of wastes as defined in 40 CFR 261.3, or [B] Those substances defined as hazardous wastes in 49 CFR 171.8 (2).
These criteria are extensively used in the classification of appropriate personal protective equipment regulations developed by OSHA. These standards developed by OSHA can provide guidance for employees who are handling or controlling a HS release (2). There is no formal interpretation of the applicability of these guidelines specifically for patient decontamination that is health care facility (HCF) based (2). See OSHA’s web site at http://www.osha.gov for access to and further clarification of these criteria.
A HS release from a terrorism event may be an agent with the potential for significant lethality such as sarin nerve gas, or it may be simply an unanticipated byproduct of the event itself such as airborne dirt and debris from a collapsed building (3). There are a wide variety of potential agents with hazardous effects that could be released either intentionally or unintentionally (as a byproduct of combustion or structure collapse) that further complicates the situation (2). The wide variety of potential terrorist agents and scenarios in which these agents could be used substantiates the need for clearly developed recommendations and guidelines for personal protection.
The disregard of appropriate personal protective equipment (PPE) as well as adequate decontamination procedures (discussed in Chapter 29) may result in injury or death not only to
the rescuer but also to surrounding persons (4,5,6). Secondary contamination of support staff and treating personnel may occur through physical contact of the patient, the patient’s secretions, and even sharing the same breathing space (4,5,6). Furthermore, the improper utilization of PPE may result in harm to the user as demonstrated in Israel during the 1991 Gulf War (7). Several reports documenting fatalities were attributed at least in part solely to the use of gas masks (no HS was released) (7,8). Thirteen people suffocated to death due to mishandling of gas masks in one report (7). Three other postoperative patients died after developing complications related to the use of full-fitting gas masks during the first missile attack on Israel in 1991 (8).
the rescuer but also to surrounding persons (4,5,6). Secondary contamination of support staff and treating personnel may occur through physical contact of the patient, the patient’s secretions, and even sharing the same breathing space (4,5,6). Furthermore, the improper utilization of PPE may result in harm to the user as demonstrated in Israel during the 1991 Gulf War (7). Several reports documenting fatalities were attributed at least in part solely to the use of gas masks (no HS was released) (7,8). Thirteen people suffocated to death due to mishandling of gas masks in one report (7). Three other postoperative patients died after developing complications related to the use of full-fitting gas masks during the first missile attack on Israel in 1991 (8).
PPE needs may vary considerably with the individual terrorist event. Factors in determining appropriate PPE include time and location of victim encounter by rescue personnel (first responders vs. emergency department staff), nature of HS (biological vs. chemical), unexpected hazards (burning PVC pipe from a fire), and available equipment. Some medical personnel will require the ability to perform sophisticated procedures such as endotracheal intubation or establishment of intravenous access. Certain levels of protection (A, B) may greatly complicate the completion of these tasks and have to be considered in the overall emergency response plan. This chapter reviews definitions of PPE and the current established recommendations for use in various situations.
PREPAREDNESS ESSENTIALS
A HS must gain entry to the body in order to cause toxicity. The two major ways chemical agents gain entry to the body is through the mucosa of the oral and respiratory tracts and by absorption through the skin (9). Biological agents also may gain entry through these pathways. Although consumption of HSs in foodstuffs or drinking water is well reported (10) and may result in toxicity, this pathway is not relevant for this chapter. The proximity of pulmonary blood to capillary membranes needed for gas exchange in the lungs provides a unique opportunity for rapid absorption of an inhaled toxin. As a result, there is considerable effort targeted to protection of the respiratory tract. Successful protection for medical personnel responding to a terrorist event depends on a careful assessment of known and unknown exposure risks and implementation of an integrated approach to respiratory and dermal protection.
The principles of respiratory protection are fairly simple. Either people can protect themselves by breathing air from a source other than the ambient atmosphere (self-contained air or oxygen supply) or they can breathe the ambient air after it has passed through some type of filter to remove the HS. Both applications are used today in different situations; however, the latter has been the more common historical and practical approach.
There are numerous obstacles to overcome when designing masks that provide effective respiratory protection. Some of the major factors that contribute to tolerability include resistance to breathing, external dead space of the equipment, and weight of the mask (11,12). Many nuclear/biological/ chemical (NBC) type masks increase inspiratory resistance by four to five times that of normal (11). Compensatory changes in increased work of respiration include prolonged inspiratory time, decreased peak inspiratory flow, and decreased minute ventilation (due to reduced tidal volume, respiratory rate, and mildly increased CO2) (11,12). A gas mask’s internal volume (approximately 300 to 500 ml, depending on size) becomes external dead space that increases inhaled CO2 concentrations (11). Ventilation must therefore be adjusted to maintain normal PaCO2 (11). The considerable variation in brain stem responsiveness to elevated PaCO2, along with the other mentioned factors, may interfere with adequate ventilation (11). Exercise increases demand for oxygen, which may worsen the limiting factors just mentioned and further interfere with ventilation (11,12).
Respirators are generally uncomfortable to wear for many other reasons. Psychophysiological responses commonly present as anxiety, respiratory distress, or hyperventilation (11). People with higher baseline amounts of anxiety have an increased likelihood of psychophysiological effects (11). This may ultimately result in impaired performance while wearing the mask or, worse, removal of the mask during an actual NBC attack (11). It is also important to reemphasize that these psychophysiological responses, the inherent obstacles to appropriate ventilation just mentioned, and increased levels of stress and anxiety (i.e., during a chemical attack drill) may be synergistic in their effects to cause harm to the patient while wearing the mask (7,8). Although there are inherent problems with respiratory protective devices (RPDs), they may indeed be lifesaving if utilized correctly in an appropriate situation.
Respirators are broadly classified into two different categories. An air-purifying respirator removes contaminants from the surrounding air (13). An atmosphere-supplying respirator (ASR) provides air from a completely different source other than from the surrounding atmosphere (13). Further classification is possible based on the type of adhesion to the face or type of alternate air source (if not from the surrounding air) (11). The covering that serves as a barrier to the surrounding atmosphere and serves as a framework to which air-purifying components can be attached covers the respiratory inlet (13). These coverings can be either tight-or loose-fitting barriers (13). Tight-fitting coverings (or face pieces) are generally made of a flexible substance such as neoprene, rubber, or silicone (13). The elastic material making up the bulk of many of these coverings is generally made of butyl rubber because silicone rubber and perfluorocarbon rubber is either permeable or fragile (9). Face-pieces commonly cover the mouth and nose only (quarter mask), cover the nose and entire chin (half mask), or cover from the hairline to below the chin (full face-piece) (13) (Fig. 28-1).
Loose-fitting coverings include a wide variety of protective materials including hoods, helmets, suits, and blouses (13). A hood is a lightweight product that covers only the head, neck, and shoulders (13). A helmet generally refers to a hood with some sort of firm headgear attached (13). A blouse covers the torso down to the waist (13).
Loose-fitting coverings include a wide variety of protective materials including hoods, helmets, suits, and blouses (13). A hood is a lightweight product that covers only the head, neck, and shoulders (13). A helmet generally refers to a hood with some sort of firm headgear attached (13). A blouse covers the torso down to the waist (13).
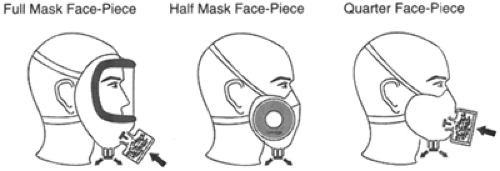 Figure 28-1. Types of Tight-Fitting Respirators by Face-Piece. Adapted from: Bollinger NJ, Schultz RH. NIOSH guide to industrial respiratory protection. National Institute of Occupational Safety and Health. Washington DC, September 1, 1987 Publication No. 87-116. Web site: http://www.cdc.gov/niosh/87-116.html. Accessed October 22, 2003. |
Air-purifying respirators can be broadly classified into particulate filtering, vapor and gas removing, and powered or nonpowered. Particulate filtering respirators generally use a fibrous material to trap the particles desired (13). The efficacy for a certain type of particulate mask depends on both particle and mask fiber velocity, composition, shape, electrical charge, and size (12). These masks are generally used for dusts, fumes, and mists (13). Many types are also effective at blocking transmission of respiratory droplets containing biological agents such as the virus that causes severe acute respiratory syndrome (SARS) and the mycobacterium that causes tuberculosis (14).
The removal of a HS by vapor- and gas-removing masks occurs by a filter container inserted into these masks. Elimination from inspired air occurs inside the filter container by inactivation through reaction by chemicals inside the filter. For example, the “hypo helmet” of 1915 removed chlorine through a reaction with sodium thiosulfate inside the filter (9), adsorption to some substance such as activated charcoal, absorption to another specific material, catalysis, or a combination of methods (9,13). Adsorption refers to the ability of the filter material (commonly referred to as the sorbent) to attract and hold the agent through physical and chemical attraction (13). Effective sorbents have large surface areas to enhance adsorption (up to 1,500 m2/g) (13). Activated charcoal is one of the most commonly used sorbents (9,13). Activated charcoal can also be impregnated with certain chemicals such as chromium and silver salts or copper oxide (9,13). This can increase the protection afforded by these masks through enhanced selective binding of a specific toxin (9,13). Catalysis refers to the utilization of a chemical to influence a chemical reaction. An example is the use of manganese oxide in a filter cartridge to speed the reaction between carbon monoxide and oxygen to make carbon dioxide (13). Absorption is similar to adsorption, except that it occurs much faster, absorbents tend to have smaller surface areas, and molecular space penetration is deeper (13). The modern C2A1 filter canister uses a pleated white filter (for aerosol removal), a layer of ASZ (impregnated with zinc) charcoal, and then a final filter to remove charcoal dust before allowing filtered air to exit the canister and be inhaled into the lungs (9). Protective masks all employ a filter layer that does not allow passage of particles and aerosols with a size greater than 3 micrometers (9). The effectiveness of all of these cartridges are commonly targeted toward specific types of agents such as organic vapors or acid gases and therefore they cannot always be safely used interchangeably (13).
These filters themselves can be inside canisters or cartridges. The major difference between the two is only due to sorbent volume (and subsequently greater adsorptive capacity). Canisters have greater volume and are often used with full face-piece coverings, commonly known as gas masks (13). Cartridges are smaller, have less sorbent, and are usually used on quarter, half, as well as full face-piece coverings (13). They are referred to as chemical cartridge respirators (CCRs) (13). Cartridges and canisters may protect against particulates as well (13).
Gas masks and CCRs can be powered or nonpowered. The term generally refers to a device that forces ambient air through the filtering device (13). Advantages of this device include cooling of the facial skin and decreased work of breathing. Disadvantages include the need for an electrical power source and potentially shorter life of the filter (because normal airflow through a nonpowered filter is only during inspiration) (13).
Atmosphere-supplying respirators (ASRs) can be classified into two major groups: self-contained breathing apparatus (SCBA) and supplied-air respirators (SARs). Each has its own unique set of advantages and disadvantages from each other as well as from the air-purifying respirators (APRs) category. Atmosphere-supplying respirators provide air from a source other than the ambient atmosphere. SCBA has the advantage of allowing increased mobility because the wearer carries a container of stored air with them. SCBA systems can be closed circuit or open circuit. A closed-circuit system utilizes 100% oxygen in a breathing bag. Oxygen is inhaled from the breathing bag through a one-way valve into the lungs. Exhalation occurs through a one-way valve, the oxygen/carbon dioxide mixture passes through a carbon dioxide scrubber that removes the carbon dioxide, and the oxygen returns to the breathing bag to be inhaled again. As oxygen is metabolized, additional oxygen is added to the breathing bag from a compressed oxygen source.
An open-circuit system is one where compressed air is inhaled through a regulator and the exhaled air is discharged into the ambient atmosphere (13). Open-circuit systems may be “demand” or “pressure demand,” and these terms generally refer to the negative- or positive-pressure environment inside the suit and the regulator piece (13). Demand-type mechanisms utilize an admission valve that only opens and supplies breathing air during inhalation (13). During exhalation, the valve closes (13). This system tends to create more of a negative-pressure system and is probably only as effective as many APRs (depending on the face-piece) (13). This characteristic makes these systems inappropriate for environments that are immediately dangerous to life and health (IDLH) (13). Pressure-demand systems utilize a similar system with the exception of a spring, which tends to hold the admission valve partially open at most times thereby providing a tendency toward a positive-pressure environment (13). Closed-circuit systems have longer use times, typically from 1 to 4 hours, whereas open-circuit systems have much larger workloads and subsequently less service time (30 to 60 minutes) (13).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


