Peritoneal/Omental Mass: Biopsy
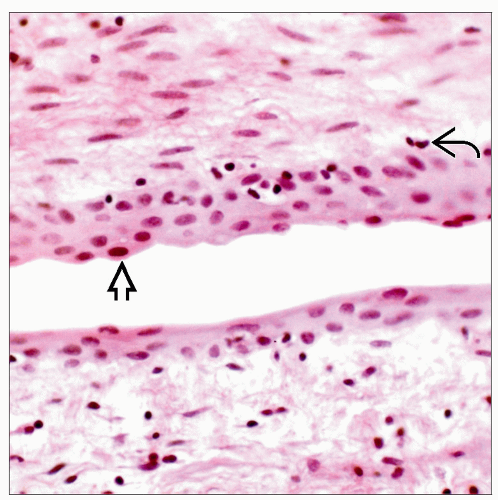
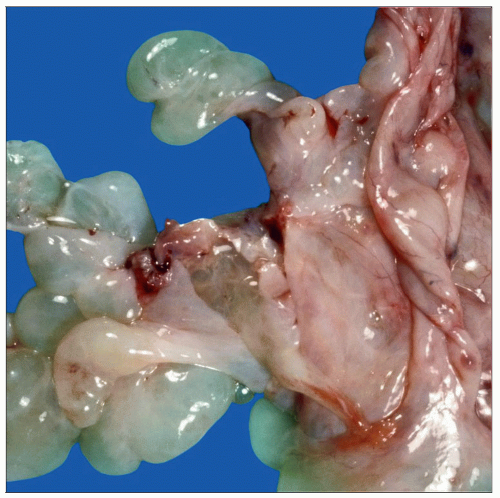 Peritoneal inclusion cysts are common incidental findings at the time of surgery. The cysts are usually translucent with a thin wall and filled with clear fluid. Larger cysts can cause symptoms. |
SURGICAL/CLINICAL CONSIDERATIONS
Goal of Consultation
Identify a mass within peritoneum as benign or malignant
Change in Patient Management
Planned surgery may be altered if malignancy is identified
If presence of malignancy is unexpected, search for primary site may be undertaken
In cases of known malignancy, the presence of metastatic disease may change type of planned surgery
For women with ovarian lesions, peritoneum or omentum may be sampled to determine if invasive implant is present
If found, the surgeon may choose to place port for chemotherapy
SPECIMEN EVALUATION
Gross
Masses are generally completely removed as small excisional biopsies
In patient with an ovarian mass, the omentum may be removed and sent for intraoperative consultation to assess the presence and types of implants
Some masses will be lymph nodes
In general, mass need not be inked as primary isolated malignancies of peritoneum are exceedingly rare
Tissue is serially sectioned
Focal lesions are identified
Size, number, color, and borders of lesions are recorded
Frozen Section
Small biopsies are completely frozen
In large samples, areas of fleshy tumor growth should be preferentially sampled
Areas of extensive necrosis or mucin should be avoided as they are often nondiagnostic
Frozen section evaluation in grossly normal specimen is low yield and generally contraindicated
MOST COMMON DIAGNOSES
Metastatic Carcinoma (Nonovarian)
If patient has history of malignancy, or known current malignancy, it is important to know type and whether or not the patient has been treated
Tumors of gastrointestinal tract often metastasize to peritoneum and have a variety of histologic presentations
Colon cancer is typically composed of glands with varying degrees of cystic dilation
Tall columnar tumor cells line glands
Extensive central “dirty” necrosis is common
Pancreatic carcinomas are often metastatic at time of surgery
Presence of mucinous glands, desmoplasia, and perineural invasion are often helpful features in identifying this tumor
Surgery is often aborted if metastatic disease is identified
Signet ring cell gastric carcinoma may metastasize widely
Small tumor cells with little cytoplasmic mucin may resemble histiocytes or lymphocytes and be difficult to identify
Enlarged cells with abundant mucin vacuoles that displace the nuclei are easier to identify
Lobular carcinoma of breast may have a strikingly similar appearance and should be included in differential diagnosis if primary is unknown
Pseudomyxoma peritonei is often secondary to an appendiceal primary
Specimen is primarily composed of mucin
Only scant foci of mucinous epithelium may be present and may not be seen on frozen section
Neuroendocrine tumors are typically composed of nests, cords, or trabeculae of tumor cells, which display vesicular (i.e., “salt and pepper”) chromatin
Cancers from nonabdominal sites also metastasize to the abdomen but are less common
Ovarian Carcinoma
In women with ovarian masses, serous tumors (borderline, low grade, and high grade) are the most likely to be associated with metastases
High-grade carcinomas frequently display striking nuclear atypia, increased mitotic activity, and widespread dissemination
Low-grade carcinomas often show destructive invasion of underlying structures but are cytologically bland compared to their high-grade counterparts
Women with tumors of low malignant potential (borderline tumors) may have extraovarian implants at time of surgery
Type of implant is important prognostic factor
Noninvasive implant (borderline tumor)
Desmoplastic: Associated with marked stromal reaction
Form smoothly contoured foci of glands surrounded by fibrous stroma
Papillae and glandular structures often present
Epithelial: Not associated with a marked stromal reaction
Circumscribed clusters of glands
May have branching papillae and detached cell clusters
Invasive implant (borderline tumor)
Definite irregular destructive invasion with desmoplasia into normal underlying tissue structures
If invasive implant is definitively identified on frozen section, surgeon may opt to place catheter for chemotherapy at time of surgery
Mesothelial Lesions
Reactive mesothelial hyperplasia is a relatively common finding and is composed of tufts or papillae lined by bland mesothelial cells
Inciting source of irritation such as peritonitis or endometriosis is often present
By definition, invasion into underlying tissue is not present
Reactive mesothelial cells can take on spindle cell morphology
Necrosis can be present, but nuclear pleomorphism is generally minimal and mitoses are not prominent
Low-grade (well-differentiated) mesotheliomas may present as cystic or papillary masses
More common in women
Generally found as incidental finding during surgery
Usually < 2 cm in size (although rare lesions are diffuse)
Papillae have simple architecture
Lining cells are bland, mitotic figures are absent to rare, and atypia should be minimal
Invasion is not present
Reactive and low-grade mesothelial lesions may be impossible to differentiate on frozen section, and extensive sampling for permanent sections should be performed
Malignant mesothelioma may present with a myriad of histologic patterns including papillary, tubulopapillary, solid, sarcomatoid, and epithelioid
Tumors are often bulky and widespread and display invasion, necrosis, increased mitotic activity, and nuclear atypia
Areas may be low grade in appearance and mimic a well-differentiated or reactive tumor
Adenomatoid Tumor
Benign proliferation of mesothelial cells
Can occur in subserosa of uterus and in the fallopian tube as well as testis and epididymis
Form firm gray-tan nodules that can be as large as 3 cm
Cells line tubular spaces or form cords
Borders can be infiltrative
May be present in smooth muscle or dense stroma
Peritoneal Inclusion Cysts
Mesothelial-lined simple cysts filled with clear fluid
Small cysts are common incidental findings
Large cysts can be symptomatic
Endometriosis
Grossly forms red to black masses (has appearance of “powder burns”)
Diagnosis requires identification of endometrial glands, endometrial-type stroma, and hemosiderin-laden macrophages (evidence of hemorrhage)
Glands may be difficult to identify in some cases
Presence of endometrial-type stroma and the stigmata of hemorrhage are consistent with endometriosis
Polypoid endometriosis forms large cystic masses in bowel wall
Pseudoxanthomatous endometriosis has central necrosis and surrounding chronic inflammation
Endosalpingiosis
Tubal-type epithelium can be found in sites away from fallopian tube such as peritoneum and lymph nodes in women
Small, scattered, simple glands with single epithelial layer lining
Lined by secretory-type cells, peg cells, and ciliated cells
Occasionally, larger cysts, simple intraluminal papillae, or cellular stratification may be present
Endocervicosis
Benign endocervical-type glands found in cul-de-sac and posterior uterine serosa
Mucinous glands display basally oriented nuclei and abundant, eosinophilic mucinous cytoplasm
Decidual Reaction
Forms white masses, plaques, or polypoid masses in peritoneum
Consists of small collections of large, polygonal, eosinophilic cells with central round nuclei
Gliomatosis
Presence of mature glial tissue on peritoneal surfaces
Ectopic tissue is identical to mature glial tissue in central nervous system
Small round nuclei
Abundant eosinophilic fibrillary cytoplasm
May be associated with ovarian teratoma
Splenosis
Often associated with prior abdominal trauma
Splenosis presents as a single accessory spleen or scattered red to brown nodules
Histologically identical to splenic parenchyma
Peritoneal Leiomyomatosis
May be primary or secondary to morcellation of uterine leiomyomas
Histologically similar to uterine leiomyomas
Composed of bland, spindled cells with cigar-shaped nuclei arranged in intersecting fascicles
Developmental Remnants
Mesonephric remnants are found near fallopian tube
Scattered glandular structures surrounded by small bundles of smooth muscle and lined by low cuboidal epithelium
Eosinophilic secretions may be present in glandular lumina
Displaced pancreatic tissue can be present in wall of small intestine
Urachal remnants occur in dome of urinary bladder
Fat Necrosis
May present as a circumscribed or irregular firm mass that may be calcified
Infarcted Epiploic Appendage
Generally round and firm with a gritty cut surface due to calcification
Fat necrosis and calcification are diagnostic findings
Nodule may be too calcified to cut on a cryostat
In such cases, presumptive diagnosis can be made based on gross appearance
Granulomatous Peritonitis
Granulomas can be found in peritoneum for many reasons
Keratin
May be due to inflammatory changes with squamous metaplasia
Also associated with ruptured mature teratomas or endometrioid adenocarcinomas with squamous differentiation
Infections
Mycobacterium tuberculosis
Fungi
Foreign material
Barium or plant material from bowel perforation
Sarcoidosis
Actinomycetes
Formation of multiple peritoneal necrotic masses closely mimics carcinoma clinically
Abscess formation with acute inflammation is typical
Sulfur granules consist of filamentous bodies
Reactive fibrosis and necrosis can be marked
Sclerosing Mesenteritis
Reactive condition that mimics malignancy
Lesion has lobular architecture divided by fibrous bands
Lobules are composed of varying amounts of fat cells (many of which are undergoing fat necrosis), chronic inflammatory cells, and scattered calcifications
Liver Capsule Lesions
Consist of proliferations of small tubules in a cellular, but not desmoplastic, stroma
Borders are circumscribed
Atypia and mitoses should be absent
Bile duct adenomas and biliary hamartomas may be biopsied when they form small white masses on liver capsule
REPORTING
Frozen Section
Known primary
If histologic features of the 2 tumors are similar, the specimen may be confidently diagnosed as metastasis
Unknown primary
In cases where primary neoplasm is undefined, reporting “adenocarcinoma, not otherwise specified” is appropriate
If characteristic histologic features are present, an attempt to identify primary site may be appropriate
For example, report “adenocarcinoma with dirty necrosis, suggestive of colonic origin”
Lesions of indeterminate malignancy
In cases of unknown malignant potential, the phrase “at least” should be utilized to convey minimum potential of the lesion
Report “at least reactive mesothelial hyperplasia, a low-grade mesothelioma cannot be completely excluded, final diagnosis deferred to permanent sections” if a lesion is not definitely benign
Benign, identifiable lesions
Common, benign lesions should be simply stated to avoid confusion (e.g., endometriosis)
Ovarian implants
If implant shows clear obvious invasion, this can be reported
If features of invasion are not definitive, it is preferable to defer classification to permanent sections
PITFALLS
Metastatic Carcinoma vs. Benign Lesions
There are many lesions that form benign glands or pseudoglands in peritoneum
If patient has known malignancy, it is very helpful to compare histologic appearances
Metastases usually closely resemble primary carcinoma
RELATED REFERENCES
1. Malpica A et al: Well-differentiated papillary mesothelioma of the female peritoneum: a clinicopathologic study of 26 cases. Am J Surg Pathol. 36(1):117-27, 2012
2. Vlachos K et al: Sclerosing Mesenteritis: Diverse clinical presentations and dissimilar treatment options. A case series and review of the literature. Int Arch Med. 4:17, 2011
3. Longacre TA et al: Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol. 29(6):707-23, 2005
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree