Parathyroidectomy
Jarrett E. Walsh
Geeta Lal
In experienced hands, parathyroidectomy is >95% successful in curing hyperparathyroidism. Hyperparathyroidism may result from the overactivity of one gland, as in the case of a parathyroid adenoma or rarely a parathyroid carcinoma, or it may result from the overactivity of multiple glands, as in the case of diffuse hyperplasia or multiple adenomas. The goal of parathyroidectomy is to remove all hyperfunctioning parathyroid tissues.
The gold standard operation for hyperparathyroidism is a bilateral neck exploration, which entails identification of all parathyroid glands. Advances including localization studies, use of intraoperative parathyroid hormone (PTH) assays, and endoscopic techniques have allowed for less invasive and more focused explorations. This chapter will focus on the standard, formal four-gland exploration. Various minimally invasive techniques in use will also be briefly discussed.
SCORE™, the Surgical Council on Resident Education, classified parathyroidectomy as an “ESSENTIAL COMMON” procedure.
STEPS IN PROCEDURE
Initial positioning and exposure are similar as those for thyroidectomy (see Chapter 6)
Standard Four-gland Exploration
Ligate middle thyroid vein and retract gland medially
Identify and preserve recurrent laryngeal nerve
Identify all parathyroid glands bilaterally before biopsy or resection
Superior parathyroid glands commonly found dorsal to recurrent laryngeal nerve and posterior to the upper thyroid capsule
Inferior parathyroid glands commonly found anterior to recurrent laryngeal nerve near lower thyroid pole and thyrothymic ligament
If single adenoma—resect
If multiglandular disease—subtotal parathyroidectomy or total parathyroidectomy and autotransplantation
Focused Exploration or Reoperation
Consider lateral incision for re-exploration
Proceed as guided by ultrasound, radioisotope studies, or intraoperative PTH levels
Close without drains
HALLMARK COMPLICATIONS
Hypoparathyroidism
Recurrent or persistent hyperparathyroidism
Injury to recurrent laryngeal nerve
Bleeding
LIST OF STRUCTURES
Adult Structures
Parathyroid glands
Superior parathyroid glands
Inferior parathyroid glands
Thyroid gland
Middle thyroid vein
Inferior thyroid artery
Superior thyroid artery
Recurrent laryngeal nerve
Thymus
Thyrothymic ligament
Mediastinum
Cricoid cartilage
Thyroid cartilage
Esophagus
Tracheoesophageal groove
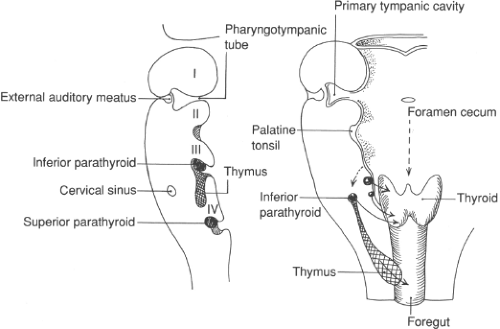
Embryologic Structures
Pharyngeal pouch III
Ventral wing
Dorsal wing
Pharyngeal pouch IV
Ventral wing
Dorsal wing
Patients requiring parathyroidectomy should be suitable candidates for general anesthesia. Special attention should be given to preoperative planning in those patients with significant hypercalcemia (i.e., calcium levels higher than 12.5 mg/dL) or marginal renal function. Specifically, this patient population may benefit from the administration of furosemide, bisphosphonates, or calcitonin to address their significant hypercalcemia, and adequate hydration is essential. Document vocal cord function by direct or indirect laryngoscopy in those already suspected of having vocal cord dysfunction (i.e., those with prior neck surgery or altered phonation), because bilateral injury may lead to airway obstruction.
Initial Exposure of Parathyroid Glands
Technical Points
Position the patient with adequate neck exposure in mind. The neck should be dorsally extended on a donut cushion with a bean bag beneath the shoulders in a modified “beach chair” position. Consider additional reverse Trendelenburg positioning of the patient to facilitate venous outflow from the cervical region during the procedure. Make an incision approximately 1 cm below the cricoid cartilage (Fig. 7.1) and carry this through the subcutaneous tissues and platysma. Develop subplatysmal flaps superiorly to the thyroid cartilage notch and inferiorly to the suprasternal notch using electrocautery. After placing a self-retaining retractor, divide the strap muscles through the midline raphe (Fig. 7.2A) and mobilize them laterally off the thyroid gland.
Dissect the sternothyroid muscle off the thyroid and prethyroidal fascia by blunt and sharp dissection, with careful attention to hemostasis. Then retract the strap muscles laterally to expose the middle thyroid vein. Ligate and divide this vein (Fig. 7.2B) and retract the thyroid lobe medially. Sometimes, several branches, rather than one middle thyroid vein, are encountered. These branches may be similarly ligated and divided. A Kitner or 2-0 silk suture placed in the surface of the thyroid gland may be used to help retract the thyroid gland medially. Develop the space between the thyroid gland and the carotid sheath bluntly or with gentle sharp dissection. Identify and protect the recurrent laryngeal nerve using a neurostimulator, if desired.
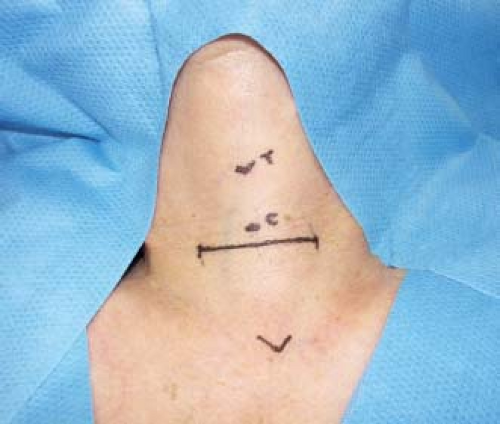 Figure 7.1 Operative photo showing anatomic landmarks and placement of the incision approximately 1 cm inferior to the cricoid cartilage (C). The thyroid cartilage is also Identified (T). |
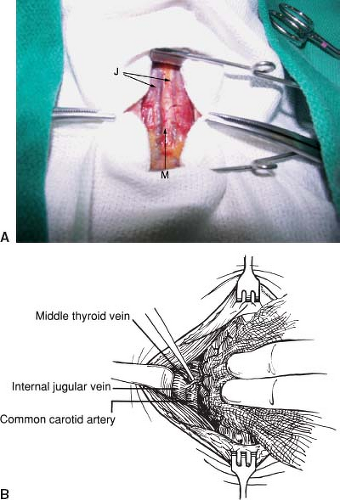 Figure 7.2 A: Operative photo showing the midline raphe (M) flanked by the anterior jugular veins (J). B: Identification and division of the middle thyroid vein. |
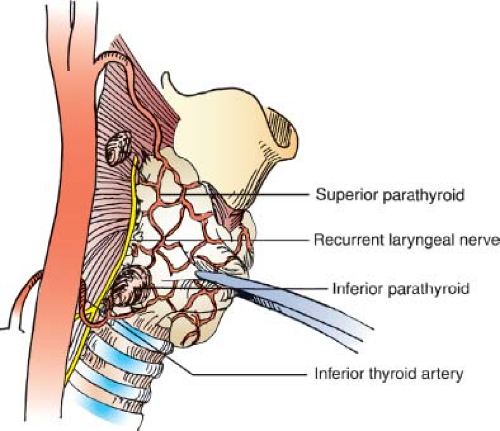
Most parathyroid glands are found within 1 cm of the junction of the inferior thyroid artery and the recurrent laryngeal nerve. The superior parathyroid glands are usually found dorsal to the recurrent laryngeal nerve and posterior to the upper thyroid capsule. The inferior parathyroid glands are usually located anterior to the recurrent laryngeal nerve near the lower thyroid pole and thyrothymic ligament. Location of glands can vary widely, reflecting differences in the degree of embryologic migration and the extent of displacement as glands enlarge.
Any fat lobule at a typical parathyroid gland location could be harboring a parathyroid gland and should be carefully evaluated. A fine, curved Jacobson hemostat and a tenotomy scissors or scalpel can be used to incise the fascia overlying a suspicious fat lobule allowing an underlying parathyroid gland to come into view, the so-called “float sign.”
Any fat lobule at a typical parathyroid gland location could be harboring a parathyroid gland and should be carefully evaluated. A fine, curved Jacobson hemostat and a tenotomy scissors or scalpel can be used to incise the fascia overlying a suspicious fat lobule allowing an underlying parathyroid gland to come into view, the so-called “float sign.”
After identification of the parathyroid glands on one side, the exploration should be continued on the contralateral side. When glands on one side are located in their normal position, the contralateral gland is usually located in a similar position. Contralateral symmetry is observed 80% of the time with superior parathyroid glands and 70% of the time with inferior parathyroid glands. Ideally in a four-gland exploration, all parathyroid glands are identified before removing any parathyroid tissue. During thyroidectomy, vessels are dissected and ligated on the surface of the thyroid gland (extrathyroidal dissection). However, during parathyroidectomy, the dissection is carried out more laterally to facilitate identification of the parathyroid glands and avoid inadvertent devascularization. As in the case of thyroidectomy, careful dissection and meticulous hemostasis are essential to prevent blood-stained tissues that can obscure parathyroid gland identification.
Anatomic Points
The middle thyroid vein is varied and is present in only about half of the patients. The vein drains into the ipsilateral internal jugular vein after traveling superficial to the common carotid artery. Division of the middle thyroid vein or veins allows mobilization of the thyroid gland medially. This allows exposure of the space between the thyroid gland and the carotid sheath (containing the carotid artery, internal jugular vein, and vagus nerve). This space contains the recurrent laryngeal nerve, the inferior thyroid artery, and the parathyroid glands.
Normal parathyroid glands are golden yellow to light brown in color. The color does vary depending on the fat and oxyphil cell content of the gland and on the gland’s vascularity. Each gland generally weighs between 40 and 50 mg and is 3 to 7 mm in size. Although the parathyroid glands usually derive their blood supply from branches of the inferior thyroid artery, branches of the superior thyroid artery supply approximately 20% of superior glands.
Most individuals (approximately 84%) have four glands, 13% to 20% have more than four glands, and 3% have fewer than four glands. The superior parathyroid glands are generally superior and dorsal to the intersection of the inferior thyroid artery and the recurrent laryngeal nerve. The inferior parathyroid glands are usually inferior and ventral to the intersection (Fig. 7.3).
Parathyroid glands are generally soft and molded by their anatomic position. Distinguishing between a normal and hypercellular gland is often difficult. Generally, hypercellular glands are larger in size (i.e., >7 mm) and darker, firmer, and more vascular. An intraoperative density test may prove useful. Hypercellular glands tend to sink when submersed in a saline solution, whereas normal glands usually float. No single characteristic is 100% reliable in distinguishing normal from hypercellular glands. The surgeon must therefore rely on a combination of factors; this gestalt recognition favors the well-trained and experienced eye.
Identification of the Superior Parathyroid Glands
Technical Points
The superior parathyroid gland generally resides at the posterior aspect of the thyroid lobe approximately 1 cm superior to the intersection of the inferior thyroid artery and the recurrent laryngeal nerve. The gland is often found within a globule of fat. Careful dissection with a Jacobson or right angle clamp of the fascial layers covering the gland usually aids in identification. Gentle probing with a Kitner often causes the gland to come into view. If differentiation between a fat lobule and gland is still in question, a fine scissors or scalpel can be used to cut a small piece from the nonhilar aspect of the gland. A parathyroid gland diffusely oozes from its cut surface where fat generally bleeds from distinct small blood vessels. The sample of tissue obtained can also be sent for frozen section analysis if there is still a question.
If after an extensive search, a superior gland has not been located, dissection should proceed posteriorly, examining the tracheoesophageal groove and retroesophageal space. The retroesophageal space can be entered and bluntly dissected by passing a finger between the esophagus and vertebral column. The operative field should be first explored visually, and then using a finger, this space should be palpated from the larynx down to the posterior mediastinum. The dissection plane
is generally posterior to the recurrent laryngeal nerve. The inferior thyroid artery can generally be preserved by carefully dissecting around it. Tracing the course of the inferior thyroid artery, which usually supplies the superior gland, may also aid in its identification. Open the carotid sheath and explore this space both visually and by palpation. If a superior gland is still not found, it may be present under the thyroid capsule or within the substance of the thyroid gland. Incise the capsule of the upper thyroid pole sharply with fine scissors, and seek a subcapsular gland. Intraoperative ultrasound of the thyroid may help in identifying an intrathyroidal parathyroid gland, particularly if an ultrasound was not obtained preoperatively. In a hyperparathyroid patient in whom all other glands have been confirmed as normocellular by frozen section, an ipsilateral thyroid lobectomy may be performed and the lobe “bread-loafed” or cut into fine sections to reveal an intrathyroidal gland. Of note, this maneuver is not necessary if a preoperative ultrasound has ruled out intrathyroid nodules.
is generally posterior to the recurrent laryngeal nerve. The inferior thyroid artery can generally be preserved by carefully dissecting around it. Tracing the course of the inferior thyroid artery, which usually supplies the superior gland, may also aid in its identification. Open the carotid sheath and explore this space both visually and by palpation. If a superior gland is still not found, it may be present under the thyroid capsule or within the substance of the thyroid gland. Incise the capsule of the upper thyroid pole sharply with fine scissors, and seek a subcapsular gland. Intraoperative ultrasound of the thyroid may help in identifying an intrathyroidal parathyroid gland, particularly if an ultrasound was not obtained preoperatively. In a hyperparathyroid patient in whom all other glands have been confirmed as normocellular by frozen section, an ipsilateral thyroid lobectomy may be performed and the lobe “bread-loafed” or cut into fine sections to reveal an intrathyroidal gland. Of note, this maneuver is not necessary if a preoperative ultrasound has ruled out intrathyroid nodules.