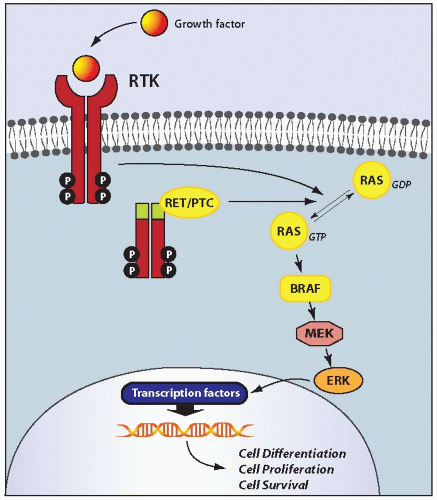
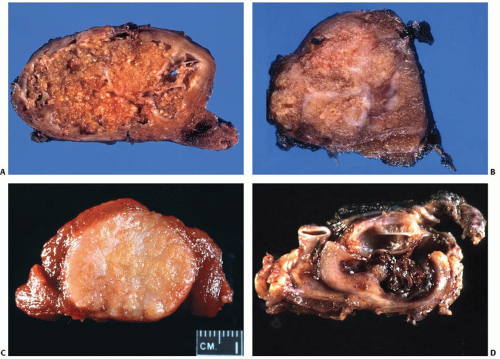
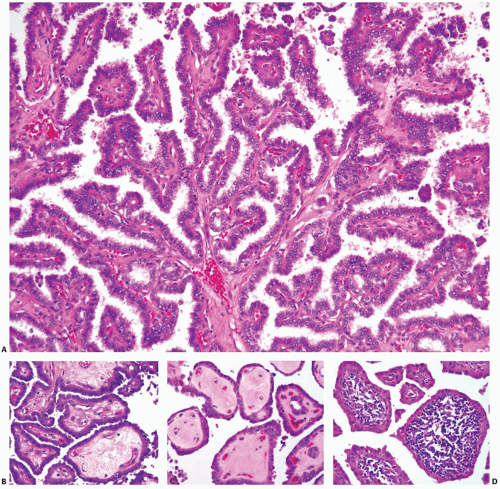
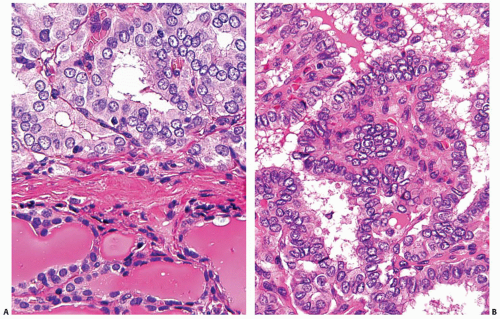
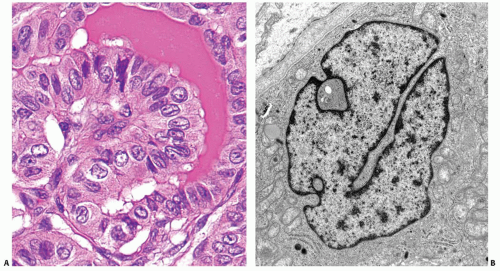
The pathogenesis of papillary carcinoma involves the perturbation of multiple signaling pathways, the most essential of which is the mitogen-activated protein kinase (MAPK) pathway that regulates cell growth, differentiation, and survival (
Fig. 11.4).
109 Activation of this pathway in thyroid cells results from point mutation of the
BRAF and
RAS genes or chromosomal rearrangement involving the
RET and
NTRK1 genes. These mutational events rarely overlap in the same tumor, and one of these alterations is found in >70% of papillary carcinomas.
110,
111,
112 Despite the common ability to activate the MAPK pathway, each of these mutations is likely to have additional and unique effects on cell transformation, as they are associated with distinct phenotypical and biologic properties of papillary carcinoma (
Table 11.2).
113
BRAF
Mutations of the
BRAF gene represent the most common genetic alteration in papillary carcinomas as they are found in 40% to 45% of these tumors. The spectrum of mutations affecting this gene includes point mutations, small in-frame deletions or insertions, and chromosomal rearrangement. The most common mutation in papillary carcinoma is a point mutation that involves a thymine to adenine substitution at nucleotide position 1799,
resulting in a valine-to-glutamate replacement at residue 600 (V600E).
110,
114 BRAF V600E comprises 98% to 99% of all
BRAF mutations found in thyroid cancer. Other alterations involve K601E point mutation and small in-frame insertions or deletions surrounding codon 600,
115,
116,
117,
118,
119 as well as
AKAP9/BRAF rearrangement.
37 The rearrangement is a paracentric inversion of chromosome 7q leading to the fusion between the portion of
BRAF gene encoding the protein kinase domain to the
AKAP9 gene.
37 All point mutations and the rearrangement lead to the activation of BRAF kinase and chronic stimulation of the MAPK pathway.
The causal role of
BRAF mutation in tumor initiation has been confirmed in transgenic mice with thyroid-specific expression of V600E.
54,
120 These animals frequently developed papillary carcinomas with microscopic features similar to tumors found in humans, although when expressed at physiologic levels in thyroid cells, the mutant BRAF apparently requires TSH stimulation to initiate papillary carcinoma development.
54BRAF V600E mutation is typically found in classic papillary carcinomas and in the tall cell variant and is rare in the follicular variant.
113,
121,
122 Tumors harboring
BRAF K601E mutation usually have the follicular variant of papillary carcinoma histology.
115,
119
As discussed in more detail later in the chapter, the presence of
BRAF V600E mutation correlates with aggressive tumor characteristics such as extrathyroidal extension, tumor recurrence, and distant metastases.
123 The association between this mutation and older patient age has also been seen in many studies.
123 BRAF association with more invasive tumor characteristics is likely due to the upregulation of expression of vascular endothelial growth factor (VEGF), matrix metalloproteinases, and other tumor cancer-promoting targets by mutant BRAF.
124,
125 The mutation may predispose to tumor dedifferentiation, as it is found in papillary carcinomas that undergo anaplastic transformation.
121,
126 These properties of mutant
BRAF are confirmed in transgenic animals, which develop papillary carcinomas with frequent wide invasion into perithyroidal tissues and progression to poorly differentiated carcinoma.
120 A process of dedifferentiation of
BRAF-mutated cancers coincides with profound deregulation of the expression of genes involved in cell adhesion and intracellular junction, providing evidence for epithelialmesenchymal transition.
127,
128
RET/PTC
RET/PTC rearrangement is another genetic alteration found in a significant proportion of papillary carcinomas. It is formed by fusion among the 3’ portion of the
RET gene, coding for the receptor tyrosine kinase (RTK), and the 5’ portion of various unrelated genes. The two most common rearrangement types,
RET/PTC1 and
RET/PTC3, are paracentric inversions because both
RET and its respective fusion partner,
H4 or
NCOA4 (ELE1; RFG), reside in the long arm of chromosome 10.
129,
130,
131,
132 RET/PTC2 and nine more recently identified types of
RET/PTC are all interchromosomal translocations (
Table 11.3). Most of these rare
RET/PTC types have been found in papillary carcinomas from patients with a history of exposure to ionizing radiation,
133,
134,
135,
136,
137,
138,
139 with the exception of the
ELKS/RET and
HOOK3/RET fusions that have been identified in tumors from patients with no radiation exposure history.
140,
141
RET is not expressed in normal thyroid follicular cells in contrast to thyroid C cells. As a consequence of
RET/PTC rearrangement, the portion of
RET coding for the tyrosine kinase domain is fused in frame with an active promoter of the fusion partner gene. As a result, the truncated RET receptor becomes constitutively expressed and activated, stimulating the MAPK signaling.
RET/PTC transforms thyroid cells in culture
142 and leads to the development of papillary carcinomas in transgenic mice, which has been shown in animals with thyroid-specific expression of
RET/PTC1 and
RET/PTC3.
143,
144,
145
The prevalence and specificity of
RET/PTC rearrangement varies dramatically in the reported series.
146,
147,
148 In part, this is due to true difference in the prevalence of this alteration in specific age groups and in individuals exposed to ionizing radiation. However, in many cases, this is due to heterogeneous distribution of this rearrangement within the tumor and variable sensitivity of the detection. This rearrangement can be present in a significant proportion of tumor cells and detected by multiple methods (
clonal RET/PTC) or can occur in a small fraction or single cells within the lesion and can be detectable only using ultrasensitive detection techniques (
nonclonal RET/PTC).
149,
150 Clonal
RET/PTC occurs in 10% to 20% of papillary thyroid carcinomas and is specific for this tumor type,
150,
151 whereas nonclonal rearrangements have been reported with a significantly higher prevalence in papillary
carcinomas and also in various other thyroid tumors and benign lesions.
146Clonal
RET/PTC is more common in papillary carcinomas from children and young adults and in patients with a history of radiation exposure. This includes individuals subjected to either accidental (mostly radioiodine) irradiation or therapeutic (mostly external beam) irradiation, as 50% to 80% of those papillary carcinomas harbor
RET/PTC.
152,
153 RET/PTC can be induced by ionizing radiation in cultured human thyroid cells and in human thyroid tissue grafted into mice.
154,
155 The rearrangement may be a direct result of misrejoining of DNA breaks induced by radiation; this may be facilitated by close spatial positioning of chromosomal regions involved in
RET/PTC generation, which can be seen by fluorescence in-situ hybridization (FISH) in normal human thyroid cells (
Fig. 11.5).
156,
157In most series of radiation-induced and sporadic tumors,
RET/PTC1 is more common. The notable expression is a population of papillary carcinomas that developed in children shortly (4 to 10 years) after radiation exposure at Chernobyl. Among those,
RET/PTC3 was the most prevalent rearrangement type.
152,
158RET/PTC-positive papillary carcinomas typically present at younger age and have classic papillary histology, a high rate of lymph node metastases, but low stage at presentation. These findings are particularly characteristic of tumors harboring
RET/PTC1.113 Among papillary carcinomas associated with radiation exposure,
RET/PTC1 is associated with classic papillary carcinoma, whereas
RET/PTC3 type is associated with the solid variant.
38 RET/PTC-positive tumors lack the predisposition for progression to poorly differentiated and anaplastic carcinomas.
159
The pathogenetic role of nonclonal
RET/PTC rearrangement, i.e.
RET/PTC detected at a very low level or in single cells within the thyroid nodule or in a gland affected by Hashimoto thyroiditis, is not clear.
147,
149 A claim that frequent detection of
RET/PTC using highly sensitive techniques in glands affected by Hashimoto thyroiditis provides evidence for multiple occult papillary carcinomas that are not detectable histologically
63,
64 cannot be accepted at this time.
67
RAS
Point mutations involving
RAS genes are found in about 10% of papillary carcinomas and affect almost exclusively the follicular variant of this tumor.
160,
161 The mutations are located at several specific sites (codons 12, 13, and 61) of the
NRAS,
HRAS, and
KRAS genes.
162,
163,
164 The mutation stabilizes the protein in its active, GTP-bound conformation, resulting in chronic stimulation of several signaling pathways, most importantly the MAPK and phosphatidylinositol-3-kinase (PI3K/AKT) pathways. In addition to strong correlation with the follicular variant histology, this mutation is also associated with more frequent tumor encapsulation, less prominent nuclear features of papillary carcinoma, and a lower rate of lymph node metastases.
113,
160 Some studies have reported an association between
RAS mutation and a higher frequency of distant metastases.
165
TRK
Rearrangement of the
NTRK1 gene, named TRK rearrangement, is the least frequent type of mutation capable of activating the MAPK pathway.
NTRK1 is located on chromosome 1q22 and encodes a cell membrane receptor with tyrosine kinase activity.
166,
167 Similar to the
RET gene,
NTRK1 is not expressed in normal thyroid follicular cells. The rearrangement juxtaposes the portion of
NTRK1 coding for the intracellular tyrosine kinase domain to the 5’ terminal sequence of one of three genes that are highly expressed in thyroid follicular cells.
167 Two of them, the
TPM3 gene
168,
169 and the
TPR gene,
170,
171 are also located on chromosome 1q, and therefore, these fusions are intrachromosomal inversions. The third fusion partner, the
TFG gene, resides on chromosome 3, and this fusion is a result of the t(1;3) translocation.
172 All fusion types lead to the expression and activation of the tyrosine kinase domain of
NTRK1. The fusion is tumorogenic for thyroid follicular cells, as
TPR/NTRK1 drives the development of papillary carcinomas in transgenic mice.
173TRK rearrangements occur in <5% of papillary carcinomas,
174,
175 although in some regions of the world the reported frequency is in the range of 10% to 15%.
167,
176,
177 All three fusion types are found with approximately similar incidence, and several tumors with
NTRK1 fused to still unknown genes have been reported.
176,
177 Approximately 5% of radiation-induced papillary carcinomas carry this rearrangement, most commonly the
NTRK1/TPM3 type.
178
Mutations in the Oncocytic Variant of Papillary Carcinoma
The oncocytic variant of papillary carcinoma reveals the characteristic nuclear features of papillary carcinoma along with cytoplasmic granularity due to accumulation of numerous and frequently abnormal mitochondria. The cause of the mitochondrial change and its relationship to the neoplastic process are not fully understood. It is likely that it may represent either a primary process induced by a distinct tumor initiation mutation or a secondary, probably compensatory change.
184,
185,
186Mutations of the
GRIM-19 (
NDUFA13) gene, which encodes a protein that regulates cell death as well as mitochondrial metabolism, occur in oncocytic thyroid tumors. In one study of 10 oncocytic papillary carcinomas, two tumors revealed somatic missense mutations and one a germline mutation of
GRIM-19.
187 The mutations may disrupt the function of this anti-apoptotic tumor suppressor gene and promote tumorigenesis. However, the prevalence of
GRIM-19 mutations in oncocytic thyroid tumors and their role in carcinogenesis remains to be fully characterized.
In some studies, a high prevalence of
RET/PTC rearrangements has been found in oncocytic variant papillary carcinomas using highly sensitive detection techniques.
188,
189,
190 The significance of these findings is not clear because these ultrasensitive methods also led to the detection of nonclonal
RET/PTC in many oncocytic follicular adenomas and carcinomas.