KEY POINTS
Neurologic surgery specializes in primarily surgical management of central, peripheral, and autonomic nervous system disorders.
Although clinical examination is paramount, neurosurgical diagnosis and treatment are aided largely by a variety of modalities, such as MRI and intracranial pressure monitoring.
The common treatment goals for traumatic brain and spinal injury are aimed at preventing secondary insults of hypoxia and hypotension.
Aneurysmal subarachnoid hemorrhage remains one of the most morbid and intensive neurosurgical diseases. Endovascular therapy is a growing technology that allows for safer securing of ruptured aneurysms.
Brain tumors can arise from primary or metastatic tissues. Treatment typically involves resection, followed by radiation and/or chemotherapy, depending on the type and grade of tumor.
Spinal instrumentation is used for surgical stabilization of many types of spinal instability, including traumatic, infectious, oncologic, and degenerative.
Infection of the nervous system is a serious and prevalent medical problem. Operative management is indicated for most conditions in which there is symptomatic compression of neural structures.
Functional neurosurgery via device implantation is a rapidly evolving discipline that has already become the standard of care in treating medically refractory Parkinson’s disease and essential tremor. A wider variety of deep brain stimulation targets will treat additional neuropsychiatric diseases.
Stereotactic radiosurgery is a powerful treatment option for intracranial disease, whether it is primary or adjunct. Gamma knife surgery can be used to treat tumors, vascular malformations, and cranial neuralgias.
OVERVIEW
Neurologic surgery provides the operative and nonoperative management (i.e., prevention, diagnosis, evaluation, treatment, critical care, and rehabilitation) of disorders of the central, peripheral, and autonomic nervous systems (ANSs). Such disorders include those of the brain, meninges, skull and skull base, and their blood supply, including surgical and endovascular treatment of disorders of the intracranial and extracranial vasculature supplying the brain and spinal cord; disorders of the pituitary gland; disorders of the spinal cord, meninges, and vertebral column, including those that may require treatment by fusion, instrumentation, or endovascular techniques; and disorders of the cranial and spinal nerves throughout their distribution.
An accurate history is the first step toward neurologic diagnosis. A history of trauma or of neurologic symptoms is of obvious interest, but general constitutional symptoms are also important. Neurologic disease may have systemic effects, while diseases of other systems may affect neurologic function. The patient’s general medical ability to withstand the physiologic stress of anesthesia and surgery should be understood. A detailed history from the patient and/or family, along with a reliable physical examination, will clarify these issues.
NEUROANATOMY
An understanding of neuroanatomy is the foundation of comprehensive neurologic examination and diagnosis. Salient features will be considered, from cephalad to caudad. The cerebral hemispheres (or telencephalon) consist of the cerebral cortex, underlying white matter, the basal ganglia, hippocampus, and amygdala. The cerebral cortex is the most recently evolved part of the nervous system. Its functions are mapped to discrete anatomic areas. The frontal areas are involved in executive function, decision making, and restraint of emotions. The motor strip, or precentral gyrus, is the most posterior component of the frontal lobes, and is arranged along a homunculus with the head inferior and lateral to the lower extremities superiorly and medially. The motor speech area (Broca’s area) lies in the left posterior inferior frontal lobe in almost all right-handed people and in up to 90% of left-handed people. The parietal lobe lies between the central sulcus anteriorly and the occipital lobe posteriorly. The postcentral gyrus is the sensory strip, also arranged along a homunculus. The rest of the parietal lobe is involved with awareness of one’s body in space and relative to the immediate environment, body orientation, and spatial relationships. The occipital lobes are most posterior. The visual cortex is arrayed along the apposing medial surfaces of the occipital lobes. The left occipital lobe receives and integrates data from the left half of each retina. A left occipital lesion would therefore result in an inability to see objects right of center. The temporal lobes lie below the sylvian fissures. The hippocampus, amygdala, and lower optic radiations (Meyer’s loops) are important components of the temporal lobe and are involved in memory, emotion, and vision, respectively. The receptive speech area (Wernicke’s area) typically is found in the area of the left posterior superior temporal lobe and inferior parietal lobe. The basal ganglia include the caudate, putamen, globus pallidus, subthalamic nucleus, substantia nigra, and nucleus accumbens. These structures are involved in the selection, activation and termination of movement, and facilitate learning of appropriate context-dependent motor behaviors.
Lying deep to the cerebral hemispheres is the diencephalon, which includes the thalamus and hypothalamus. The thalamus is a key processor and relay circuit for most motor and sensory information traveling to or from cortex. The hypothalamus regulates homeostasis via the autonomic and neuroendocrine systems.
The brain stem consists of the midbrain (mesencephalon), pons (metencephalon), and medulla (myelencephalon). Longitudinal fibers run through the brain stem, carrying motor and sensory information between the cerebral hemispheres and spinal cord. The corticospinal tract is the major motor tract, while the medial lemniscus and spinothalamic tracts are the major sensory tracts. The nuclei of cranial nerves III through XII are also located within the brain stem. These nerves relay the motor, sensory, and special sense functions of the eye, face, mouth, and throat.
The cerebellum arises from the dorsal aspect of the brain stem. It integrates somatosensory, vestibular, and motor information for coordination and timing of movement. Midline, or vermian, lesions lead to truncal ataxia. Lateral, or hemispheric, lesions lead to tremor and dyscoordination in the extremities.
The ventricular system is the cerebrospinal fluid (CSF)–containing contiguous space inside the brain, continuous with the subarachnoid space outside the brain. The paired lateral ventricles consist of temporal, occipital, and frontal horns, as well as the main body. CSF travels from each lateral ventricle through the foramina of Monroe to the third ventricle, located between the left and right thalami. CSF then drains through the cerebral aqueduct to the fourth ventricle within the brain stem. The foramen of Magendie (midline) and paired foramina of Luschka (lateral) drain to the subarachnoid space. The approximate CSF volume in an average adult is 150 mL, and the choroid plexus produces approximately 500 mL of CSF per day.
The spinal cord starts at the bottom of the medulla and extends caudally through the spinal canal to the first lumbar vertebra, approximately. Motor tracts (efferent pathways) continue from the brain stem down via the lateral and anterior corticospinal tracts to anterior horn cells, and then exit via ventral nerve roots. Sensory information (afferent pathways) enters via dorsal nerve roots, travels cranially via the dorsal columns (proprioception and fine touch) or spinothalamic tract (pain and temperature), and into the brain stem. Paired nerves exit the spinal cord at each level. There are 31 pairs: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal.
The dorsal and ventral nerve roots at each level fuse to form mixed motor-sensory spinal nerves and spread through the body to provide innervation to muscles and sensory organs. The C5–T1 spinal nerves intersect in the brachial plexus and divide to form the main nerve branches to the arm, including the median, ulnar, and radial nerves. The L2–S4 spinal nerves intersect in the lumbosacral plexus, and divide to form the main nerve branches to the leg, including the femoral and sciatic nerves.
The principal motor tract of the spinal cord is the corticospinal tract. It is a two-neuron path, including an upper motor neuron and a lower motor neuron. The upper motor neuron cell body is located within the motor strip of the cerebral cortex. The axon travels through the internal capsule to the brain stem, decussates at the brain stem–spinal cord junction, and travels down the contralateral corticospinal tract to the lower motor neuron in the anterior horn at the appropriate level. The lower motor neuron axon then travels via peripheral nerves to its target muscle. Damage to upper motor neurons typically results in hyperreflexia and mild atrophy Damage to lower motor neurons results in flaccidity and significant atrophy.
The two major sensory tracts are three-neuron pathways. Fine touch and proprioceptive signals enter the spinal cord via the dorsal root ganglia and then ascend ipsilaterally via the dorsal columns. Then they synapse and decussate in the lower medulla, travel up the contralateral medial lemniscus to make a second synapse in the thalamus, and then finally ascend to the sensory cortex. Pain and temperature fibers first synapse in the dorsal horn of the spinal cord at their entry level, decussate, and then travel up the contralateral spinothalamic tracts to the thalamus. The second synapse occurs in the thalamus, and the output axons ascend to the sensory cortex.
The aforementioned motor and sensory tracts together constitute the somatic nervous system. In addition to this system, the ANS is the other constituent of the nervous system. The ANS carries messages for homeostasis and visceral regulation from the central nervous system (CNS) to target structures such as arteries, veins, the heart, sweat glands, and the digestive tract.1 CNS control of the ANS arises particularly from the hypothalamus and the nucleus of the tractus solitarius. The ANS is divided into the sympathetic, parasympathetic, and enteric systems. The sympathetic system drives the “fight or flight” response, using epinephrine to increase heart rate, blood pressure, blood glucose, and temperature, as well as to dilate the pupils. It arises from the thoracolumbar spinal segments. The parasympathetic system promotes the “rest and digest” state, and uses acetylcholine to maintain basal metabolic function under nonstressful conditions. Parasympathetic fibers arise from cranial nerves III, VII, IX, and X, and from the second to fourth sacral segments. The enteric nervous system controls the complex synchronization of the digestive tract, especially the pancreas, gallbladder, and small and large bowels. It can run autonomously but is regulated by the sympathetic and parasympathetic systems.
NEUROLOGIC EXAMINATION
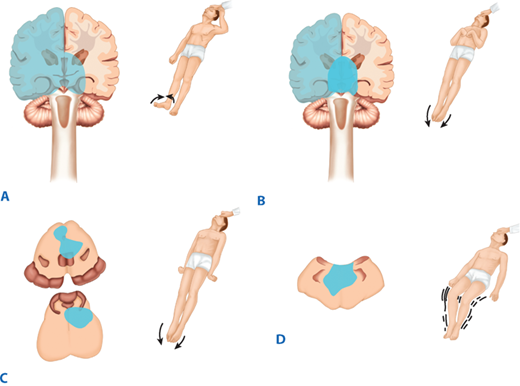
The neurologic examination is divided into several components, and generally is done from head to toe. First, one must assess mental status. A patient may be awake, lethargic (will follow commands and answer questions, but then returns to sleep), stuporous (difficult to arouse), or comatose (no purposeful response to voice or pain). Cranial nerves may be thoroughly tested in the awake patient, but pupil reactivity, eye movement, facial symmetry, and gag are the most relevant measures when mental status is impaired. Motor testing is based on maximal effort of major muscle groups in those able to follow commands, while assessing for amplitude and symmetry of movement to deep central pain may be all that is possible for stuporous patients. Table 42-1 details scoring for motor assessment tests. Characteristic motor reactions to pain in patients with depressed mental status include withdrawal from stimulus, localization to stimulus, flexor (decorticate) posturing, extensor (decerebrate) posturing, or no reaction (in order of worsening pathology). Figure 42-1 diagrams the clinical patterns of posturing. This forms the basis of determining the Glasgow Coma Scale (GCS) motor score, as detailed in Table 42-2. Light touch, proprioception, temperature, and pain testing may be useful in awake patients but is often impossible without good cooperation. It is critical to document sensory patterns in spinal cord injury (SCI) patients. Muscle stretch reflexes should be examined. Often comparing left to right or upper extremity to lower extremity reflexes for symmetry is the most useful for localizing a lesion. Check for ankle-jerk clonus or up-going toes (Babinski’s test). Presence of either is pathologic and signifies upper motor neuron disease.
| MOTOR RESPONSE | VERBAL RESPONSE | EYE-OPENING RESPONSE | |||
|---|---|---|---|---|---|
| Obeys commands | 6 | Oriented | 5 | Opens spontaneously | 4 |
| Localizes to pain | 5 | Confused | 4 | Opens to speech | 3 |
| Withdraws from pain | 4 | Inappropriate words | 3 | Opens to pain | 2 |
| Flexor posturing | 3 | Unintelligible sounds | 2 | No eye opening | 1 |
| Extensor posturing | 2 | No sounds | 1 | ||
| No movement | 1 |
Figure 42-1.
Patterns of motor responses associated with various lesions. A. Left hemispheric lesion with right hemiplegia and left localization. B. Deep cerebral/thalamic lesion with bilateral flexor posturing. C. Midbrain or pontine lesion with bilateral extensor posturing. D. Medullary lesion with general flaccidity. (Adapted with permission from Rengachary SS: Impaired consciousness, in Rengachary SS, Ellenbogen RG [eds]: Principles of Neurosurgery, 2nd ed. Edinburgh; New York: Elsevier Mosby, 2005. Copyright Elsevier.)
Plain X-rays of the skull may demonstrate fractures, osteolytic or osteoblastic lesions, or pneumocephaly (air in the head). The use of skull plain films has decreased given the rapid availability and significantly increased detail of head computed tomography (CT) scans. Plain films of the cervical, thoracic, and lumbar spine are used to assess for evidence of bony trauma or soft tissue swelling suggesting fracture. Spinal deformities and osteolytic or osteoblastic pathologic processes also will be apparent. The shoulder girdle usually poses problems in visualizing the cervicothoracic junction clearly.
The noncontrast CT scan of the head is an extremely useful diagnostic tool in the setting of new focal neurologic deficit, decreased mental status, or trauma. It is rapid and almost universally available in hospitals in the United States. Its sensitivity allows for the detection of acute hemorrhage. A contrast-enhanced CT scan will help show neoplastic or infectious processes. In the current era, contrast CT generally is used for those patients who cannot undergo magnetic resonance imaging (MRI) scanning due to pacemakers or metal in the orbits. Fine-slice CT scanning of the spine is helpful for defining bony anatomy and pathology, and is usually done after an abnormality is seen on plain films, or because plain films are inadequate (especially to visualize C7 and T1 vertebrae). Finally, high-speed multislice scanners, combined with timed-bolus contrast injections, allow CT angiography. A thin-slice axial scan is obtained during the passage of contrast through the cerebral arteries and reconstructed in three dimensions to assess for vascular lesions. CT angiography does not reliably detect lesions, such as cerebral aneurysms <3 mm across, but can provide detailed morphologic data of larger lesions. Newer, multislice scanner technology is approaching the resolution of conventional angiography.
MRI provides excellent imaging of soft tissue structures in the head and spine. It is a complex and evolving science. Several of the most clinically useful MRI sequences are worth describing. T1 sequences made before and after gadolinium administration are useful for detecting neoplastic and infectious processes. T2 sequences facilitate assessment of lesion-associated edema in the brain and neural compression in the spine by the presence or absence of bright T2 CSF signals. Diffusion-weighted images can detect ischemic stroke earlier than CT. Fine-slice time-of-flight axial images can be reformatted in three dimensions to build MRI angiograms and MRI venograms. MRI angiograms can detect stenosis of the cervical carotid arteries or intracranial aneurysms >3 mm in diameter. MRI venograms can assess the dural venous sinuses for patency or thrombosis.
Transarterial catheter-based angiography remains the gold standard for evaluation of vascular pathology of the brain and spine. The current state of the art is biplanar imaging to reduce dye load and facilitate interventional procedures. Digital subtraction technologies minimize bony interference in the resultant images. Bilateral carotid arteries and bilateral vertebral arteries may be injected and followed through arterial, capillary, and venous phases for a complete cerebral angiogram.
Electromyography and nerve conduction studies (EMG/NCS) are useful for assessing the function of peripheral nerves. EMG records muscle activity in response to a proximal stimulation of the motor nerve. NCS record the velocity and amplitude of the nerve action potential. EMG/NCS typically is performed approximately 3 to 4 weeks after an acute injury, as nerves distal to the injury continue to transmit electrical impulses normally until degeneration of the distal nerve progresses.
The most reliable monitor, always, is an alert patient with a reliable neurologic examination. If a reliable neurologic examination is not possible due to the presence of brain injury, sedatives, or paralytics, or if there is active and unstable intracranial pathology, invasive monitoring is required. There are several methods of monitoring intracranial physiology. The three described below are bedside intensive care unit (ICU) procedures that allow for continuous monitoring. All three procedures involve making a small hole in the skull with a hand-held drill. They generally are placed in the right frontal region to minimize the neurologic impact of possible complications such as hemorrhage.
An external ventricular drain is also known as a ventriculostomy. A perforated plastic catheter is inserted into the frontal horn of the lateral ventricle. An uninterrupted fluid column through a rigid tube allows transduction of intracranial pressure (ICP). CSF also can be drained to reduce ICP or sampled for laboratory studies.
An intraparenchymal fiber-optic pressure transducer is commonly referred to as a bolt. A threaded post locks securely into the hole made in the skull, and holds the fiber-optic catheter in place. A bolt allows ICP monitoring only, but it is smaller and less invasive than a ventriculostomy, and may be associated with fewer complications, although the data do not clearly support this.
The brain tissue oxygen sensor is a recent development that has already demonstrated a mortality benefit in traumatic brain injury patients.2 This sensor is part of a bolt, which is screwed into the skull in the same manner as the bolt described previously, however, is engineered to hold a pressure sensor, oxygen sensor, and brain temperature sensor. The oxygen sensor catheter has an electrochemical oxygen–tension sensitive membrane. Patients with severe brain injury due to trauma or aneurysmal hemorrhage may benefit from placement of these three sensors in addition to a ventriculostomy to drain CSF for control of ICP. Such monitoring requires two twist-drill holes, which may be placed on adjacent or opposite sides of the head.
NEUROLOGIC AND NEUROSURGICAL EMERGENCIES
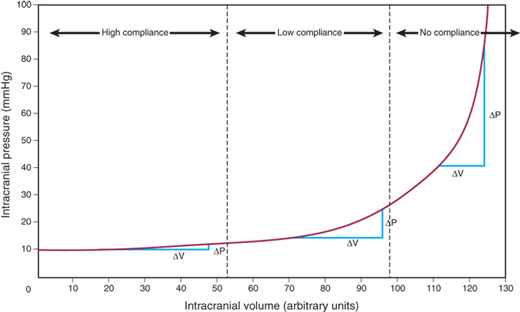
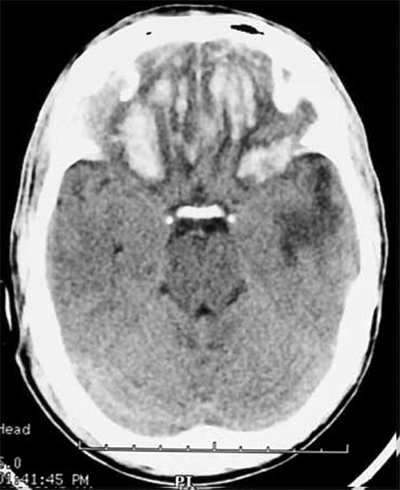
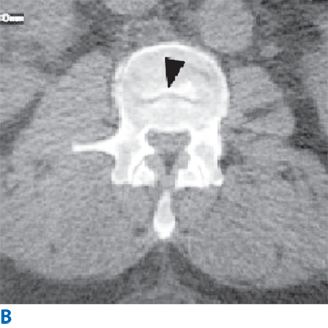
ICP normally varies between 4 and 14 mmHg. Sustained ICP levels above 20 mmHg can injure the brain. The Monro-Kellie doctrine states that the cranial vault is a rigid structure, and therefore, the total volume of the contents determines ICP. The three normal contents of the cranial vault are brain tissue, blood, and CSF. The brain’s contents can expand due to swelling from traumatic brain injury (TBI), stroke, or reactive edema. Blood volume can increase by extravasation to form a hematoma, or by reactive vasodilation in a hypoventilating, hypercarbic patient. CSF volume increases in the setting of hydrocephalus. Figure 42-2 demonstrates the classic CT findings of hydrocephalus. The addition of a lesion, such as a tumor or abscess, also will increase ICP. The pressure-volume curve depicted in Fig. 42-3 demonstrates a compensated region with a small ΔP/ΔV, and an uncompensated region with large ΔP/ΔV. In the compensated region, increased volume is offset by decreased volume of CSF and blood.
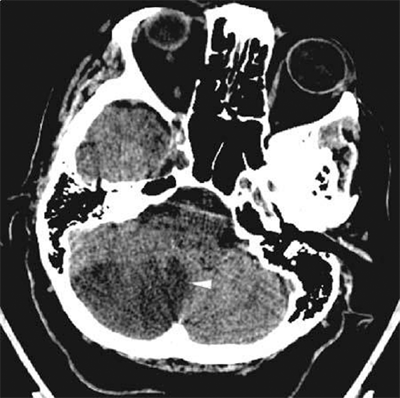
Figure 42-2.
Head computed tomography scan demonstrating hydrocephalus. The third ventricle (3rd) is widened and rounded, the anterior horns of the lateral ventricles are plump, and pressure-driven flow of cerebrospinal fluid into brain parenchyma adjacent to the ventricles is seen (arrowhead). This is known as transependymal flow of cerebrospinal fluid.
Figure 42-3.
Pressure-volume curve demonstrating the effect of changing the volume of intracranial contents on intracranial pressure. Note the compensated zone, with little change of pressure with change of volume, and the uncompensated zone, with significant change of pressure with change of volume. (Adapted with permission from Morton R, Ellenbogen RG: Intracranial consciousness, in Ellenbogen RG et al (eds): Principles of Neurosurgery, 3rd ed. Philadelphia: Elsevier Saunders, 2012, p 313. Copyright Elsevier.)
Increased ICP can injure the brain in several ways. Focal mass lesions cause shift and herniation. Temporal lesions push the uncus medially and compress the midbrain. This phenomenon is known as uncal herniation. The posterior cerebral artery (PCA) passes between the uncus and midbrain and may be occluded, leading to an occipital infarct. Masses higher up in the hemisphere can push the cingulate gyrus under the falx cerebri. This process is known as subfalcine herniation. The anterior cerebral artery (ACA) branches run along the medial surface of the cingulate gyrus and may be occluded in this case, leading to medial frontal and parietal infarcts. Diffuse increases in pressure in the cerebral hemispheres can lead to central, or transtentorial, herniation. Increased pressure in the posterior fossa can lead to upward central herniation or downward tonsillar herniation through the foramen magnum. Uncal, transtentorial, and tonsillar herniation can cause direct damage to the brain stem. Figure 42-4 diagrams patterns of herniation.
Figure 42-4.
Schematic drawing of brain herniation patterns. 1. Subfalcine herniation. The cingulate gyrus shifts across midline under the falx cerebri. 2. Uncal herniation. The uncus (medial temporal lobe gyrus) shifts medially and compresses the midbrain and cerebral peduncle. 3. Central transtentorial herniation. The diencephalon and midbrain shift caudally through the tentorial incisura. 4. Tonsillar herniation. The cerebellar tonsil shifts caudally through the foramen magnum. (Reproduced with permission from Cohen DS, Quest DO: Increased intracranial pressure, brain herniation and their control, in Wilkins RH, Rengachary SS [eds]: Neurosurgery, 2nd ed. New York: McGraw Hill, 1996, p 349.)
Patients with increased ICP, or intracranial hypertension, often will present with headache, nausea, vomiting, and progressive mental status decline. Cushing’s triad is the classic presentation of intracranial hypertension, bradycardia, and irregular respirations. Focal neurologic deficits such as hemiparesis may be present if there is a focal mass lesion causing the problem. Patients with these symptoms should undergo an immediate head CT and rapid neurosurgical evaluation.
Initial management of intracranial hypertension includes airway protection and adequate ventilation. A bolus of mannitol up to 1 g/kg causes free water diuresis, increased serum osmolality, and extraction of water from the brain. The effect is delayed by about 20 minutes and has a transient benefit. Driving serum osmolality above 300 mOsm/L is of indeterminate benefit and can have deleterious cardiovascular side effects, such as hypovolemia that leads to hypotension and decreased brain perfusion. A ventriculostomy and/or craniectomy may be needed for definitive decompression.
It is critical to note that lethargic or obtunded patients often have decreased respiratory drive. This causes the partial pressure of arterial carbon dioxide (Paco2) to increase, resulting in cerebral vasodilation and worsening of intracranial hypertension. This cycle causes a characteristic “crashing patient,” who rapidly loses airway protection, becomes apneic, and herniates. Emergent intubation and ventilation to reduce Paco2 to roughly 35 mmHg can reverse this process.
The posterior fossa (brain stem and cerebellum) requires special consideration because the volume of the posterior fossa within the cranial vault is small. Posterior fossa lesions such as tumors, hemorrhage or stroke can cause mass effect that can rapidly kill the patient in two ways. Occlusion of the fourth ventricle can lead to acute obstructive hydrocephalus, raised ICP, herniation, and eventually death. This mass effect can also lead directly to brain stem compression (Fig. 42-5). Symptoms of brain stem compression include hypertension, agitation, and progressive obtundation, followed rapidly by brain death. A patient exhibiting any of these symptoms needs an emergent neurosurgical evaluation for possible ventriculostomy or suboccipital craniectomy (removal of the bone covering the cerebellum). This situation is especially critical, as expeditious decompression can lead to significant functional recovery.
Figure 42-5.
Maturing cerebellar stroke seen as a hypodense area in the right cerebellar hemisphere (arrowhead) on head computed tomography in a patient with rapidly progressing obtundation 2 days after the initial onset of symptoms. Swelling of the infarcted tissue causes posterior fossa mass effect. The fourth ventricle is obliterated and not visible, and the brain stem is being compressed.
Patients presenting with acute focal neurologic deficits at a clearly defined time of onset (i.e., when the patient was last seen in a normal state of health) must be evaluated as rapidly as possible. An emergent head CT scan should be done. The study is often normal, because CT changes from ischemic stroke may take up to 24 hours to appear (Fig. 42-6). A patient with a clinical diagnosis of acute stroke <3 hours old, without hemorrhage on CT, may be a candidate for thrombolytic therapy with tissue plasminogen activator (tPA). An emergent MRI is helpful but not always diagnostically necessary.
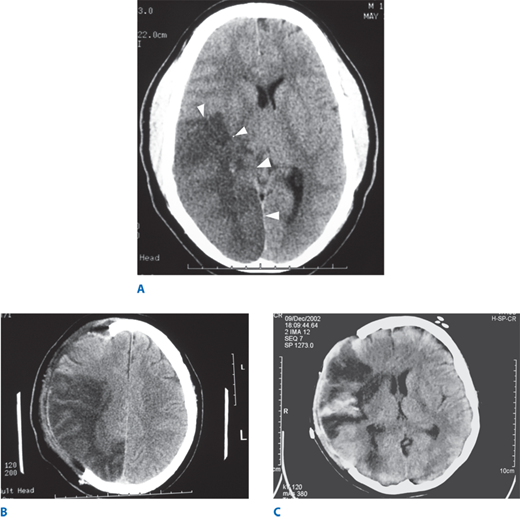
Figure 42-6.
A. Head computed tomography scan of a patient with a 4-day-old stroke that occluded the right middle cerebral and posterior cerebral arteries. The infarcted tissue is the hypodense (dark) area indicated by the arrowheads. The patient presented with left-sided weakness and left visual field loss, but then became less responsive, prompting this head computed tomography. Note the right-to-left midline shift. B. Same patient status post decompressive right hemicraniectomy. Note the free expansion of swollen brain outside the normal confines of the skull. C. Patient with a right middle cerebral artery ischemic stroke with areas of hemorrhagic conversion, seen as hyperdense (bright) areas within the infarcted tissue. This patient also required hemicraniectomy for severe mass effect. Note the lack of midline shift postoperatively.
A seizure is defined as an uncontrolled synchronous organization of neuronal electrical activity. A new-onset seizure often signifies an irritative mass lesion in the brain, particularly in adults, in whom tumors commonly present with seizure. Patients with traumatic intracranial hemorrhage are at risk for seizure. In addition to airway and ventilatory problems, a seizing patient is also at risk for neural excitotoxicity if the activity is prolonged, such as in status epilepticus. Any patient with a new-onset seizure should have imaging of the brain after the seizure is controlled and the patient is resuscitated.
TRAUMA
Trauma is the leading cause of death in children and young adults; however, the incidence of death and disability from trauma has been slowly decreasing. This decline is partly attributable to increased awareness of safety devices such as seat belts and motorist helmets. Nonetheless, trauma remains a major cause of morbidity and mortality, and it can affect every major organ system in the body. The three main areas of neurosurgical focus are: traumatic brain injury (TBI), spinal cord injury (SCI), and peripheral nerve injury.
The initial assessment of the trauma patient includes the primary survey, resuscitation, secondary survey, and definitive care. Neurosurgical evaluation begins during the primary survey with the determination of the GCS score (usually referred to simply as the GCS) for the patient. The GCS is determined by adding the scores of the best responses of the patient in each of three categories. The motor score ranges from 1 to 6, verbal from 1 to 5, and eyes from 1 to 4. The GCS therefore ranges from 3 to 15, as detailed in Table 42-2. Tracheal intubation or severe facial or eye swelling can impede verbal and eye responses. In these circumstances, the patient is given the score of 1 with a modifier, such as verbal “1T” where T = tube.
Blunt or penetrating trauma to the head can cause injury to the densely vascularized scalp, and significant blood loss can result. Direct pressure initially controls the bleeding, allowing close inspection of the injury. If a simple laceration is found, it should be copiously irrigated and closed primarily. If the laceration is short, a single-layer, percutaneous suture closure will suffice. If the laceration is long or has multiple arms, the patient may need debridement and closure in the operating room, with its superior lighting and wider selection of instruments and suture materials. Careful reapproximation of the galea will provide a more secure closure and better hemostasis. Blunt trauma also can cause crush injury with subsequent tissue necrosis. These wounds require debridement and consideration of advancement flaps to cover the defect.
The usual classification system for bony fractures may be applied to the skull. The fracture may be characterized by skull X-rays or head CT.3 A closed fracture is covered by intact skin. An open, or compound, fracture is associated with disrupted overlying skin. The fracture lines may be single (linear); multiple and radiating from a point (stellate); or multiple, creating fragments of bone (comminuted). Closed skull fractures do not normally require specific treatment. Open fractures require repair of the scalp and operative debridement. Indications for craniotomy include depression greater than the cranial thickness, intracranial hematoma, and frontal sinus involvement.4 Skull fractures generally indicate that a significant amount of force was transmitted to the head and should increase the suspicion for intracranial injury. Fractures that cross meningeal arteries can cause rupture of the underlying vessels and subsequent epidural hematoma (EDH) formation.
Depressed skull fractures may result from a focal injury of significant force. The inner and outer cortices of the skull are disrupted, and a fragment of bone is pressed in toward the brain in relation to adjacent intact skull. The fragment may overlap the edge of intact bone, or may plunge completely below the level of adjacent normal skull. The inner cortex of the bone fragments often has multiple sharp edges that can lacerate dura, brain, and vessels. Craniotomy is required to elevate the fracture, repair dural disruption, and obtain hemostasis in these cases (Fig. 42-7). However, fractures overlying dural venous sinuses require restraint. Surgical exploration can lead to life-threatening hemorrhage from the lacerated sinus.
Figure 42-7.
A. Bone-window axial head computed tomography (CT) of a patient who presented aphasic after being struck with the bottom of a beer bottle. CT demonstrates a depressed skull fracture in the left posterior temporoparietal area. B. Brain-window axial head CT demonstrating intraparenchymal hematoma caused by laceration of cortical vessels by the edge of the fractured bone. Arrowhead indicates traumatic subarachnoid hemorrhage in the sylvanian fissure.
Fractures of the skull base are common in head-injured patients, and they indicate significant impact. They are generally apparent on routine head CT, but should be evaluated with dedicated fine-slice coronal-section CT scan to document and delineate the extent of the fracture and involved structures. If asymptomatic, they require no treatment. Skull base fractures requiring intervention include those with an associated cranial nerve deficit or CSF leak. A fracture of the temporal bone, for instance, can damage the facial or vestibulocochlear nerve, resulting in vertigo, ipsilateral deafness, or facial paralysis. A communication may be formed between the subarachnoid space and the middle ear, allowing CSF drainage into the pharynx via the eustachian tube or from the ear (otorrhea). Extravasation of blood results in ecchymosis behind the ear, known as Battle’s sign. A fracture of the anterior skull base can result in anosmia (loss of smell from damage to the olfactory nerve), CSF drainage from the nose (rhinorrhea), or periorbital ecchymoses, known as raccoon eyes.
Copious clear drainage from the nose or ear makes the diagnosis of CSF leakage obvious. Often, however, the drainage may be discolored with blood or small in volume if some drains into the throat. The halo test can help differentiate. Allow a drop of the fluid to fall on an absorbent surface such as a facial tissue. If blood is mixed with CSF, the drop will form a double ring, with a darker center spot containing blood components surrounded by a light halo of CSF. If this test is indeterminate, the fluid can be sent for beta-2 transferrin testing, a carbohydrate-free isoform of transferrin exclusively found in the CSF.
Many CSF leaks will heal with elevation of the head of the bed for several days. A lumbar drain can augment this method. A lumbar drain is a catheter placed in the lumbar CSF cistern to decompress the cranial vault and allow the defect to heal by eliminating normal hydrostatic pressure. There is no proven efficacy of antibiotic coverage for preventing meningitis in patients with CSF leaks.
Traumatic cranial neuropathies generally can be managed conservatively, with documentation of the extent of impairment and signs of recovery. Patients with traumatic facial nerve palsies may benefit from a course of steroids, although their benefit is unproven. Patients with facial nerve palsy of abrupt onset, who do not respond to steroids within 48 to 72 hours, may be considered for surgical decompression of the petrous portion of the facial nerve. Patients also may present with delayed-onset facial nerve palsy. Again, steroids are used and surgery can be considered, with mixed results.
Closed head injury (CHI) is the most common type of TBI and a significant cause of morbidity and mortality in the United States. There are two important factors that affect the outcome of CHI in general. The initial impact causes the primary injury, defined as the immediate injury to neurons from transmission of the force of impact. The long, delicate axons of the neurons can shear as they undergo differential acceleration or deceleration along their projecting pathways. Prevention strategies, such as wearing helmets, remain the best means to decrease disability from primary injury. Subsequent neuronal damage due to the sequelae of trauma is referred to as secondary injury. Hypoxia, hypotension, hydrocephalus, intracranial hypertension, thrombosis, and intracranial hemorrhage may all be mechanisms of secondary injury. One focus of basic research in TBI, critical care medicine, and neurosurgical intervention is to decrease the effects of secondary injury.
The Brain Trauma Foundation’s most recent summary of management recommendations for TBI patients was published in 2007 and is endorsed by the American Association of Neurological Surgeons, Congress of Neurological Surgeons, and the World Health Organization. The guidelines standardize the care of these patients with the hope of improving outcomes. Some of the common patterns of CHI, including concussion, contusion, and diffuse axonal injury, are discussed in Types of Closed Head Injury.5
The initial evaluation of a trauma patient remains the same whether or not the primary surveyor suspects head injury. The first three elements of the ABCDs of resuscitation—airway, breathing, and circulation—must be assessed and stabilized. Hypoxia and hypotension are known to worsen outcome in TBI (due to secondary injury), making cardiopulmonary stabilization critical. Patients who cannot follow commands require intubation for airway protection and ventilatory control. The fourth element, assessment of “D,” for disability, is undertaken next. Motor activity, speech, and eye opening can be assessed in a few seconds and a GCS score assigned.
The following is an example of how a primary surveyor may efficiently assess disability and GCS: Approach the patient and enter his or her field of view. Observe whether the patient is visually attentive. Clearly command: “Tell me your name.” Then ask the patient to lift up two fingers on each side sequentially, and wiggle the toes. A visually or verbally unresponsive patient should be assessed for response to peripheral stimuli such as nail-bed pressure, or deep central painful stimulation, such as a firm, twisting pinch of the sensitive supraclavicular skin. Watch for eye opening and movement of the extremities, whether purposeful or reflexive. Assess the verbal response. The motor, verbal, and eye-opening scores may be correctly assigned using this rapid examination. An initial assessment of the probability of significant head injury can be made, assuming that pharmacologic and toxic elements have not obscured the examination. The surveyor must also take note of any external signs of head injury, including bleeding from the scalp, nose, or ear, or deformation of the skull or face.
Several medical steps may be taken to minimize secondary injury and the systemic consequences of head injury. Patients with a documented CHI and evidence of intracranial hemorrhage or a depressed skull fracture should receive a 17-mg/kg phenytoin loading dose, followed by 1 week of therapeutic maintenance phenytoin, typically 300 to 400 mg/d. Phenytoin prophylaxis has been shown to decrease the incidence of early posttraumatic seizures.6 There is no evidence to support long-term use of prophylactic antiepileptic agents. Blood glucose levels should be closely monitored by free blood sugar checks and controlled with sliding scale insulin. Fevers also should be evaluated and controlled with antipyretics, as well as source-directed therapy when possible. Hyperglycemia and hyperthermia are toxic to injured neurons and contribute to secondary injury. Head-injured patients have an increased prevalence of peptic ulceration and GI bleeding. Peptic ulcers occurring in patients with head injury or high ICP are referred to as Cushing’s ulcers. Ulcer prophylaxis should be used. Compression stockings or athrombic pumps should be used when the patient cannot be mobilized rapidly for prophylaxis of deep venous thrombosis.
TBI can be classified as mild, moderate, or severe. For patients with a history of head trauma, classification is as follows: severe head injury if the GCS score is 3 to 8, moderate head injury if the GCS score is 9 to 12, and mild head injury if the GCS score is 13 to 15. Many patients present to emergency rooms and trauma bays with a history of TBI. A triage system must be used to maximize resource utilization while minimizing the chance of missing occult or progressing injuries.
TBI patients who are asymptomatic, who have only headache, dizziness, or scalp lacerations, and who did not lose consciousness, have a low risk for intracranial injury and may be discharged home without a head CT scan.7,8 Head-injured patients who are discharged should be sent home with reliable family or friends who can observe the patient for the first postinjury day. Printed discharge instructions, which describe monitoring for confusion, persistent nausea, weakness, or speech difficulty, should be provided to the caretaker. The patient should return to the emergency department for evaluation of such symptoms.
Patients with a history of altered consciousness, amnesia, progressive headache, skull or facial fracture, vomiting, or seizure have a moderate risk for intracranial injury and should undergo a prompt head CT. If the CT is normal, and the neurologic examination has returned to baseline (excluding amnesia of the event), then the patient can be discharged to the care of a responsible adult, again with printed criteria for returning to the emergency room. Otherwise the patient must be admitted for a 24-hour observation period.
Patients with depressed consciousness, focal neurologic deficits, penetrating injury, depressed skull fracture, or changing neurologic examination have a high risk for intracranial injury. These patients should undergo immediate head CT and admission for observation or intervention as needed.
A concussion is defined as temporary neuronal dysfunction following nonpenetrating head trauma. The head CT is normal, and deficits resolve over minutes to hours. Definitions vary; some require transient loss of consciousness, while others include patients with any alteration of mental status. Memory difficulties, especially amnesia of the event, are very common. Concussions may be graded. One method is the Colorado grading system.9 Head trauma patients with confusion only are grade 1, patients with amnesia are grade 2, and patients who lose consciousness are grade 3. Studies have shown that the brain remains in a hypermetabolic state for up to a week after injury. The brain is also much more susceptible to injury from even minor head trauma in the first 1 to 2 weeks after concussion. This is known as second-impact syndrome, and patients should be informed that, even after mild head injury, they might experience memory difficulties or persistent headaches.
A contusion is a bruise of the brain, and occurs when the force from trauma is sufficient to cause breakdown of small vessels and extravasation of blood into the brain. The contused areas appear bright on CT scan, as seen in Fig. 42-8. The frontal, occipital, and temporal poles are most often involved. The brain sustains injury as it collides with rough, bony surfaces. Contusions themselves rarely cause significant mass effect as they represent small amounts of blood in injured parenchyma rather than coherent blood clots. Edema may develop around a contusion, causing mass effect. Contusions may enlarge or progress to frank hematoma, particularly during the first 24 hours. Contusions also may occur in brain tissue opposite the site of impact. This is known as a contre-coup injury. These contusions result from deceleration of the brain against the skull.
Diffuse axonal injury is caused by damage to axons throughout the brain, due to rotational acceleration and then deceleration. Axons may be completely disrupted and then retract, forming axon balls. Small hemorrhages can be seen in more severe cases, especially on MRI. Hemorrhage is classically seen in the corpus callosum and the dorsolateral midbrain.
These injuries are complex and must be evaluated individually. The two main subtypes are missile (e.g., due to bullets or fragmentation devices) and nonmissile (e.g., due to knives or ice picks). Some general principles apply. If available, skull X-rays and CT scans are useful in assessing the nature of the injury. Cerebral angiography must be considered if the object passes near a major artery or dural venous sinus. Operative exploration is necessary to remove any object extending out of the cranium, as well as for debridement, irrigation, hemostasis, and definitive closure. Small objects contained within brain parenchyma are often left in place to avoid iatrogenic secondary brain injury. Antibiotics are given to decrease the chances of meningitis or abscess formation. High-velocity missile injuries (from high-powered hunting rifles or military weapons) are especially deadly, because the associated shock wave causes cavitary tissue destruction of an area that is much larger than the projectile itself. Projectiles that penetrate both hemispheres or traverse the ventricles are almost universally fatal.
The various traumatic intracranial hematomas contribute to death and disability secondary to head injury. Hematomas can expand rapidly and cause brain shift and subsequent herniation. Emergent neurosurgical evaluation and intervention often are necessary.
EDH is the accumulation of blood between the skull and the dura. EDH usually results from arterial disruption, especially of the middle meningeal artery. The dura is adherent to bone, and some pressure is required to dissect between the two. EDH has a classic, three-stage clinical presentation that is probably seen in only 20% of cases. The patient is initially unconscious from the concussive aspect of the head trauma. The patient then awakens and has a “lucid interval,” while the hematoma subclinically expands. As the volume of the hematoma grows, the decompensated region of the pressure-volume curve is reached, ICP increases, and the patient rapidly becomes lethargic and herniates. Uncal herniation from an EDH classically causes ipsilateral third nerve palsy and contralateral hemiparesis.
On head CT the blood clot is bright, biconvex in shape (lentiform), and has a well-defined border that usually respects cranial suture lines. An EDH is typically found over the convexities but may rarely occur in the posterior fossa as well.
Open craniectomy for evacuation of the congealed clot and hemostasis generally is indicated for EDH. Patients who meet all of the following criteria may be managed conservatively: clot volume <30 cm3, maximum thickness <1.5 cm, and GCS score >8.10 Prognosis after successful evacuation is better for EDH than subdural hematoma (SDH). EDHs are associated with lower-energy trauma with less resultant primary brain injury. Good outcomes may be seen in 85% to 90% of patients, with rapid CT scan and intervention.11
An acute SDH is the result of an accumulation of blood between the arachnoid membrane and the dura. Acute SDH usually results from venous bleeding, typically from tearing of a bridging vein running from the cerebral cortex to the dural sinuses. The bridging veins are subject to stretching and tearing during acceleration/deceleration of the head, because the brain shifts in relation to the dura, which firmly adheres to the skull. Elderly and alcoholic patients are at higher risk for acute SDH formation after head trauma due to brain atrophy.
On head CT scan, the clot is bright or mixed-density, crescent-shaped (lunate), may have a less distinct border, and does not cross the midline due to the presence of the falx. Most SDHs occur over the cerebral hemispheres, but they may also occur between the hemispheres or layer over the tentorium.
Open craniotomy for evacuation of acute SDH is indicated for any of the following: thickness >1 cm, midline shift >5 mm, or GCS drop by two or more points from the time of injury to hospitalization. Nonoperatively managed hematomas may stabilize and eventually reabsorb, or evolve into chronic SDHs.12 This management requires frequent neurologic examinations until the clot stabilizes based on serial head CT scans.
The prognosis for functional recovery is significantly worse for acute SDH than EDH because it is associated with greater primary injury to brain parenchyma from high-energy impacts. Prompt recognition and intervention minimizes secondary injury. The elderly patients with low admission GCS, or high postoperative ICP do poorly, with as few as 5% attaining functional recovery.13
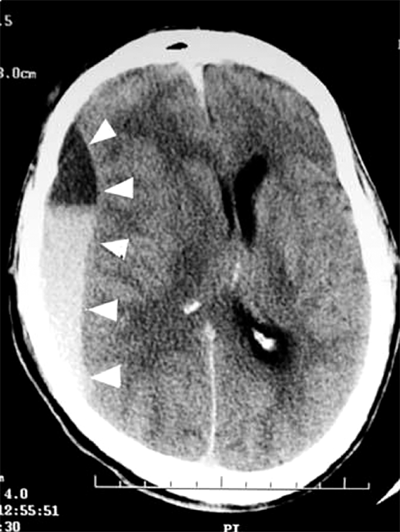
Chronic SDH is a collection of blood breakdown products that is at least 2 to 3 weeks old. Acute hematomas are bright white (hyperdense) on CT scan for approximately 3 days, after which they fade to isodensity with brain, and then to hypodensity after 2 to 3 weeks. A true chronic SDH will be nearly as dark as CSF on CT. Traces of white are often seen due to small, recurrent hemorrhages into the collection. These small bleeds may expand the collection enough to make it symptomatic. This phenomenon is referred to as an acute-on-chronic SDH. Figure 42-9 demonstrates the CT appearance of an acute-on-chronic SDH. Vascularized membranes form within the hematoma as it matures. These membranes may be the source of acute hemorrhage.
Figure 42-9.
Head computed tomography scan of an elderly patient with progressing left hemiplegia and lethargy, demonstrating an acute-on-chronic subdural hematoma. History revealed that the patient sustained a fall 4 weeks before presentation. Arrowheads outline the hematoma. The acute component is slightly denser and is seen as the hyperdense area in the dependent portion.
Chronic SDHs often occur in patients without a clear history of head trauma, as they may arise from minor head injury. Alcoholics, the elderly, and patients on anticoagulation are at higher risk for developing chronic SDH. Patients may present with headache, seizure, confusion, contralateral hemiparesis, or coma.
A chronic SDH >1 cm or any symptomatic SDH should be surgically drained. Unlike acute SDH, which consists of a thick, congealed clot, chronic SDH typically consists of a viscous fluid with the texture and dark brown color reminiscent of motor oil. A simple burr hole can effectively drain most chronic SDHs. However, the optimal treatment of chronic SDH remains controversial.14 Most authorities agree that burr hole drainage should be attempted first to obviate the risks of formal craniotomy. A single burr hole placed over the dependent edge of the collection can be made, and the space copiously irrigated until the fluid is clear. A second, more anterior burr hole can then be placed if the collection does not drain satisfactorily due to containment by membranes. The procedure is converted to open craniotomy if the SDH is too congealed for irrigation drainage, the complex of membranes prevents effective drainage, or persistent hemorrhage occurs that cannot be reached with bipolar cautery through the burr hole. The required surgical prepping and draping are always performed to allow simple conversion to craniotomy, and the scalp incision and burr holes are placed to allow easy incorporation into larger skin flaps.
There are various strategies to prevent reaccumulation of blood. Subdural or subgaleal drains may be left in place for 1 to 2 days. Mild hydration and bedrest with the head of the bed flat may encourage brain expansion. High levels of inspired oxygen may help draw nitrogen out of the cavity. Regardless of the strategy used, follow-up head CT scans are required postoperatively and approximately 1 month later to document resolution.
Isolated hematomas within the brain parenchyma are most often associated with hypertensive hemorrhage or arteriovenous malformations (AVMs). Bleeding may occur in a contused area of brain. Mass effect from developing hematomas may present as a delayed neurologic deficit. Delayed traumatic intracerebral hemorrhage is most likely to occur within the first 24 hours. Patients with contusion on the initial head CT scan should be reimaged 24 hours after the trauma to document stable pathology. Indications for craniotomy include: any clot volume >50 cm3 or a clot volume >20 cm3 with referable neurologic deterioration (GCS 6–8) and associated midline shift >5 mm or basal cistern compression.15
Trauma to the head or neck may cause damage to the carotid or vertebrobasilar systems. Generally, dissection refers to violation of the vessel wall intima. Blood at arterial pressures can then open a plane between the intima and media, within the media, or between the media and adventitia. The newly created space within the vessel wall is referred to as the false lumen. Tissue or organs supplied by dissected vessels may subsequently be injured in several ways. Expansion of the hematoma within the vessel wall can lead to narrowing of the true vessel lumen and reduction or cessation of distal blood flow. Slow-flowing or stagnant blood within the false lumen exposed to thrombogenic vessel wall elements may thrombose. Pieces of thrombus may then detach and cause distal embolic arterial occlusion. Also, the remaining partial-thickness vessel wall may rupture, damaging adjacent structures.
Traumatic dissection may occur in the carotid artery (anterior circulation) or the vertebral or basilar arteries (posterior circulation). Dissections may be extradural or intradural. Intradural dissection can present with subarachnoid hemorrhage (SAH). Traditional angiography remains the basis of diagnosis and characterization of arterial dissection. Angiographic abnormalities include stenosis of the true lumen, or “string-sign,” visible intimal flaps, and the appearance of contrast in the false lumen. Four-vessel cerebral angiography should be performed when suspicion of dissection exists.
Historically, patients with documented arterial dissection have been anticoagulated with heparin and then warfarin to prevent thromboembolic stroke. Trauma patients often have concomitant absolute or relative contraindications to anticoagulation, complicating management. Antiplatelet therapy is often implemented in lieu of full anticoagulation, however, there is no randomized clinical trial comparing the two therapies.16 Consider surgical or interventional techniques for persisting embolic disease and for vertebral dissections presenting with SAH. Surgical options include vessel ligation and bypass grafting. Interventional radiology techniques include stenting and vessel occlusion. Occlusion techniques require sufficient collateral circulation to perfuse the vascular territory previously supplied by the occluded vessel.
Carotid dissection may result from neck extension combined with lateral bending to the opposite side, or trauma from an incorrectly placed shoulder belt tightening across the neck in a motor vehicle accident. Extension or bending stretches the carotid over the bony transverse processes of the cervical vertebrae, while seat belt injuries cause direct trauma. Symptoms of cervical carotid dissection include contralateral neurologic deficit from brain ischemia, headache, and ipsilateral Horner’s syndrome from disruption of the sympathetic tracts ascending from the stellate ganglion on the surface of the carotid artery. The patient may complain of a bruit.
Traumatic vessel wall injury to the portion of the carotid artery running through the cavernous sinus may result in a carotid-cavernous fistula (CCF). This creates a high-pressure, high-flow pathophysiologic blood flow pattern. CCFs classically present with pulsatile proptosis (the globe pulses outward with arterial pulsation), retro-orbital pain, and decreased visual acuity or loss of normal eye movement (due to damage to cranial nerves III, IV, and VI as they pass through the cavernous sinus). Symptomatic CCFs should be treated to preserve eye function. Fistulae may be closed by balloon occlusion using interventional neuroradiology techniques. Fistulae with wide necks are difficult to treat and may require total occlusion of the parent carotid artery.
Vertebrobasilar dissection may result from sudden rotation or flexion/extension of the neck, chiropractic manipulation, or a direct blow to the neck. Common symptoms are neck pain, headache, and brain stem stroke or SAH. The risks and benefits of aspirin therapy are unclear when a vertebral dissection extends intracranially. The theoretically increased friability of the vessel wall may increase the risk of SAH when coupled with an antiplatelet agent. Consultation of a stroke neurologist is recommended in this situation.
Brain death occurs when there is an absence of signs of brain stem function or motor response to deep central pain in the absence of pharmacologic or systemic medical conditions that could impair brain function.
A neurologist, neurosurgeon, or intensivist generally performs the clinical brain death examination. Two examinations consistent with brain death 12 hours apart, or one examination consistent with brain death followed by a consistent confirmatory study generally is sufficient to declare brain death (see below). Hospital regulations and local laws regarding documentation should be followed closely.
Establish the absence of complicating conditions before beginning the examination. The patient must be normotensive, euthermic, and oxygenating well. The patient may not be under the effects of any sedating or paralytic drugs.
Documentation of no brain stem function requires the following: nonreactive pupils; lack of corneal blink, oculocephalic (doll’s eyes), oculovestibular (cold calorics) reflexes; and loss of drive to breathe (apnea test). The apnea test demonstrates no spontaneous breathing even when Paco2 is allowed to rise above 60 mmHg.
Deep central painful stimuli are provided by bilateral forceful twisting pinch of the supraclavicular skin and pressure to the medial canthal notch. Pathologic responses such as flexor or extensor posturing are not compatible with brain death. Spinal reflexes to peripheral pain, such as triple flexion of the lower extremities, are compatible with brain death.
Confirmatory studies are performed after a documented clinical examination consistent with brain death. A study consistent with brain death may obviate the need to wait 12 hours for a second examination. This is especially important when the patient is a potential organ donor, as brain-dead patients often have progressive hemodynamic instability. Lack of cerebral blood flow consistent with brain death may be documented by cerebral angiography or technetium radionuclide study. A “to-and-fro” pattern on transcranial Doppler ultrasonography indicates no net forward flow through the cerebral vasculature, consistent with brain death. An electroencephalogram (EEG) documenting electrical silence has been used, but generally is not favored because there is often significant artifact which impairs interpretation.
The spine is a complex biomechanical structure. The spine provides structural support for the body as the principal component of the axial skeleton, while protecting the spinal cord and nerve roots. Trauma may fracture bones or cause ligamentous disruption. Often, bone and ligament damage occur together. Damage to these elements reduces the strength of the spine and may cause instability, which compromises both supportive and protective functions. Spine trauma may occur with or without neurologic injury.
Neurologic injury from spine trauma is classified as either incomplete or complete. If there is some residual motor or sensory neurologic function below the level of the lesion, as assessed by clinical examination, the injury is defined as incomplete.17 A patient with complete neurologic dysfunction persisting 24 hours after injury has a very low probability of return of function in the involved area.
Neurologic injury from spine trauma may occur immediately or in delayed fashion. Immediate neurologic injury may be due to direct damage to the spinal cord or nerve roots from penetrating injuries, especially from stab wounds or gunshots. Blunt trauma may transfer sufficient force to the spine to cause acute disruption of bone and ligament, leading to subluxation, which is a shift of one vertebral element in relation to the adjacent level. Subluxation decreases the size of the spinal canal and neural foramina and causes compression of the cord or roots. Such neural impingement can also result from retropulsion of bone fragments into the canal during a fracture. Transection, crush injury, and cord compression impairing perfusion are mechanisms leading to SCI. Delayed neurologic injury may occur during transportation, examination of an improperly immobilized patient, or during a hypotensive episode.
Trauma causes a wide variety of injury patterns in the spine due to its biomechanical complexity. A mechanistic approach facilitates an understanding of the patterns of injury, as there are only a few types of forces that can be applied to the spine. Although these forces are discussed individually, they often occur in combination. Several of the most common injury patterns are then presented to illustrate the clinical results of these forces applied at pathologically high levels.
Bending the head and body forward into a fetal position flexes the spine. Flexion loads the spine anteriorly (the vertebral bodies) and distracts the spine posteriorly (the spinous process and interspinous ligaments). High flexion forces occur during front-end motor vehicle collisions, and backward falls when the head strikes first. Arching the neck and back extends the spine. Extension loads the spine posteriorly and distracts the spine anteriorly. High extension forces occur during rear-end motor vehicle collisions (especially if there is no headrest), frontward falls when the head strikes first, or diving into shallow water.
Force applied along the spinal axis (axial loading) compresses the spine. Compression loads the spine anteriorly and posteriorly. High compression forces occur when a falling object strikes the head or shoulders, or when landing on the feet, buttocks, or head after a fall from height. A pulling force in line with the spinal axis distracts the spine. Distraction unloads the spine anteriorly and posteriorly. Distraction forces occur during a hanging, when the chin or occiput strikes an object first during a fall, or when a passenger submarines under a loose seat belt during a front-end motor vehicle collision.
Force applied tangential to the spinal axis rotates the spine. Rotation depends on the range of motion of intervertebral facet joints. High rotational forces occur during off-center impacts to the body or head or during glancing automobile accidents.
Certain patterns of injury resulting from combinations of the previously mentioned forces occur commonly and should be recognized during plain film imaging of the spine. Always completely evaluate the spine. A patient with a spine injury at one level has a significant risk for additional injuries at other levels.
The cervical spine is more mobile than the thoracolumbar spine. Stability comes primarily from the multiple ligamentous connections of adjacent vertebral levels. Disruption of the cervical ligaments can lead to instability in the absence of fracture. The mass of the head transmits significant forces to the cervical spine during abrupt acceleration or deceleration, increasing risk for injury.
A Jefferson fracture is a bursting fracture of the ring of C1 (the atlas) due to compression forces. There are usually two or more fractures through the ring of C1. The open-mouth odontoid view may show lateral dislocation of the lateral masses of C1. The rule of Spence states that 7 mm or greater combined dislocation indicates disruption of the transverse ligament. The transverse ligament stabilizes C1 with respect to C2. Jefferson fractures dislocated <7 mm usually are treated with a rigid collar, while those dislocated 7 mm or greater usually are treated with a halo vest. Surgical intervention is not indicated.
The odontoid process, or dens, is the large ellipse of bone arising anteriorly from C2 (the axis) and projecting up through the ring of C1 (the atlas). Several strong ligaments connect the dens to C1 and to the base of the skull. Odontoid fractures usually result from flexion forces. Odontoid fractures are classified as type I, II, or III. A type I fracture involves the tip only. A type II fracture passes through the base of the odontoid process. A type III fracture passes through the body of C2. Types II and III are considered unstable and should be externally immobilized or fused surgically. Surgery often is undertaken for widely displaced fractures (poor chance of fusing) and for those that fail external immobilization. Type I fractures usually fuse with external immobilization only.
Traditionally considered a hyperextension/distraction injury from placement of the noose under the angle of the jaw, hangman’s fractures also may occur with hyperextension/compression, as with diving accidents, or hyperflexion. The injury is defined by bilateral C2 pars interarticularis fractures. The pars interarticularis is the bone between superior and inferior facet joints. Thus, the posterior bony connection between C1 and C3 is lost. Hangman’s fractures heal well with external immobilization. Surgery is indicated if there is spinal cord compression or after failure of external immobilization.
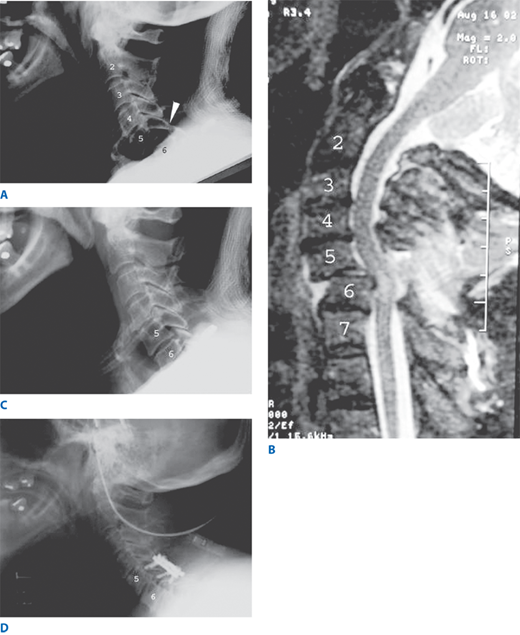
The facet joints of the cervical spine slope forward. In a hyperflexion injury, the superior facet can “jump” over the inferior facet of the level above if the joint capsule is torn. Hyperflexion/rotation can cause a unilateral jumped facet, whereas hyperflexion/distraction leads to bilateral jumped facets. Patients with unilateral injury usually are neurologically intact. Those with bilateral injury, however, typically suffer from spinal cord damage, since the anteroposterior diameter of the spinal canal is compromised by bilateral injury, leading to spinal cord compression (Fig. 42-10).
Figure 42-10.
A. Lateral cervical spine X-ray of an elderly woman who struck her head during a backward fall. Arrowhead points to jumped facets at C5–C6. Note the anterior displacement of the C5 body with respect to the C6 body. B. Sagittal T2-weighted magnetic resonance imaging of the same patient, revealing compromise of the spinal canal and compression of the cord. Note the bright signal within the cord at the level of compression, indicating spinal cord injury. C. Lateral cervical spine X-ray of same patient after application of cervical traction and manual reduction. Note restoration of normal alignment. D. Lateral cervical spine X-ray after posterior cervical fusion to restabilize the C5–C6 segment of the spine.
The thoracic spine is stabilized significantly by the rib cage. The lumbar spine has comparatively large vertebrae. Thus, the thoracolumbar spine has a higher threshold for injury than the cervical spine. A three-column model is useful for categorizing thoracolumbar injuries.18 The anterior longitudinal ligament and the anterior half of the vertebral body constitute the anterior column. The posterior half of the vertebral body and the posterior longitudinal ligament constitute the middle column. The pedicles, facet joints, laminae, spinous processes, and interspinous ligaments constitute the posterior column.
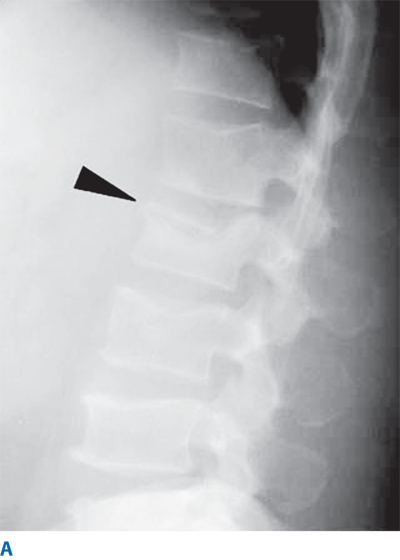
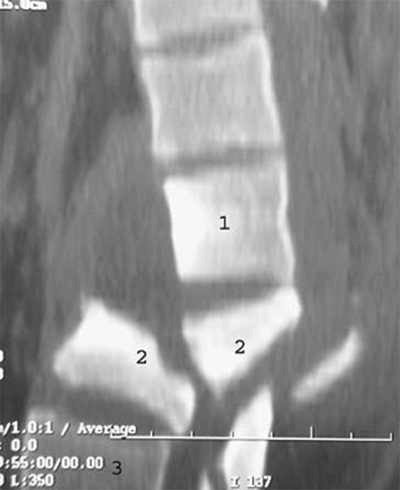
Compression fracture is a compression/flexion injury causing failure of the anterior column only. It is stable and not associated with neurologic deficit, although the patient may still have significant pain (Fig. 42-11).
Figure 42-11.
A. Lateral lumbar spine X-ray showing a compression fracture of L2. Arrowhead points to anterior wedge deformity. Note the posterior wall of the vertebral body has retained normal height and alignment. B. Axial computed tomography scan through the same fracture. Arrowhead demonstrates a transverse discontinuity in the superior endplate of the L2 body.
Burst fracture is a pure axial compression injury causing failure of the anterior and middle columns. It is unstable, and perhaps half of patients have neurologic deficit due to compression of the cord or cauda equina from bone fragments retropulsed into the spinal canal.
Chance fracture is a flexion-distraction injury causing failure of the middle and posterior columns, sometimes with anterior wedging. Typical injury is from a lap seat-belt hyperflexion with associated abdominal injury. It often is unstable and associated with neurologic deficit.
Fracture-dislocation is failure of the anterior, middle, and posterior columns caused by flexion/distraction, shear, or compression forces. Neurologic deficit can result from retropulsion of middle column bone fragments into the spinal canal, or from subluxation causing decreased canal diameter (Fig. 42-12).
The possibility of a spine injury must be considered in all trauma patients. A patient with no symptoms referable to neurologic injury, a normal neurologic examination, no neck or back pain, and a known mechanism of injury unlikely to cause spine injury is at minimal risk for significant injury to the spine. Victims of moderate or severe trauma, especially those with injuries to other organ systems, usually fail to meet these criteria or cannot be assessed adequately. The latter often is due to impaired sensorium or significant pain. Because of the potentially catastrophic consequences of missing occult spine instability in a neurologically intact patient, a high level of clinical suspicion should govern patient care until completion of clinical and radiographic evaluation.
The trauma patient should be kept on a hard flat board with straps and pads used for immobilization. A hard cervical collar is kept in place. These steps minimize forces transferred through the spine, and therefore decrease the chance of causing dislocation, subluxation, or neural compression during transport to the trauma bay. The patient is then moved from the board to a flat stretcher. The primary survey and resuscitation are completed. Physical examination and initial X-rays follow.
For the examination, approach the patient as described in the section on Neurologic Examination earlier in this chapter. Evaluation for spine or SCI is easier and more informative in awake patients. If the patient is awake, ask if he or she recalls details of the nature of the trauma, and if there was loss of consciousness, numbness, or inability to move any or all limbs. Assess motor function by response to commands or pain, as appropriate. Assess pinprick, light touch, and joint position, if possible. Determining the anatomically lowest level of intact sensation can pinpoint the level of the lesion along the spine. Testing sensation in an ascending fashion will allow the patient to better discern the true stimulus as opposed to determine when it is extinguished. Document muscle stretch reflexes, lower sacral reflexes (i.e., anal wink and bulbocavernosus), and rectal tone.
The American Spinal Injury Association provides a method of classifying patients with spine injuries. The classification indicates completeness and level of the injury and the associated deficit. A form similar to that shown in Fig. 42-13 should be available in the trauma bay and completed for any spine injury patient. The association also has worked to develop recommendations and guidelines to standardize the care of SCI patients in an effort to improve the quality of care.