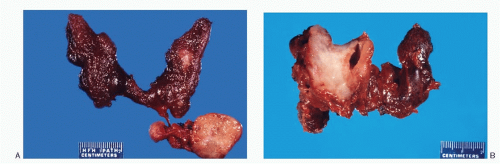
The sporadic form of medullary carcinoma occurs as a unilateral, well-defined, nonencapsulated solitary nodule variable in size, ranging from <1 cm to several centimeters, and may replace the entire lobe (
Fig. 8.2A,B). Its cut surface is tan-white to gray or pink, usually flat. The consistence is soft to firm, at times gritty, without areas of hemorrhage and necrosis. Calcification may be present. The hereditary forms are usually multifocal and bilateral (
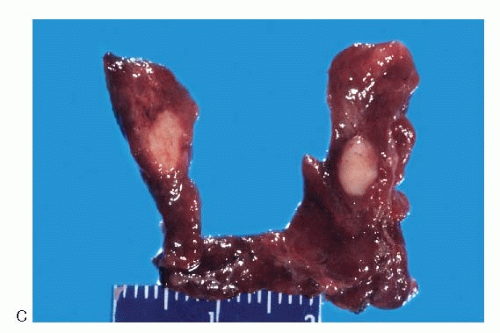
Fig. 8.2C). Their gross appearance is similar to the sporadic medullary carcinomas.
Like many neuroendocrine tumors, the medullary thyroid carcinomas present an extremely wide spectrum of morphologic patterns that varies from tumor to tumor and within the same tumor. They also share morphologic similarities with other neuroendocrine tumors such as paragangliomas, carcinoid tumors, and small cell carcinomas. In addition, medullary carcinomas mimic not only several types of thyroid follicular cell derived tumors, such as follicular adenomas including hyalinizing trabecular adenoma, follicular carcinoma, papillary, insular, Hürthle cell, or anaplastic carcinomas, but also a wide array of nonthyroid neoplasms as well as nonneoplastic entities as described in differential diagnoses (see
Table 8.4) The histologic patterns of medullary carcinomas (
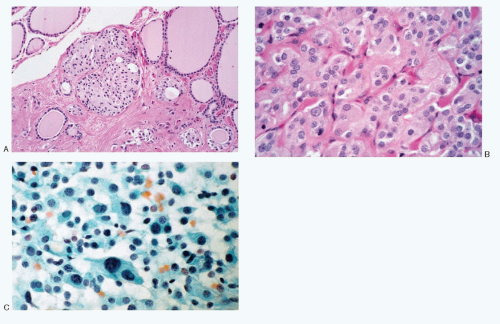
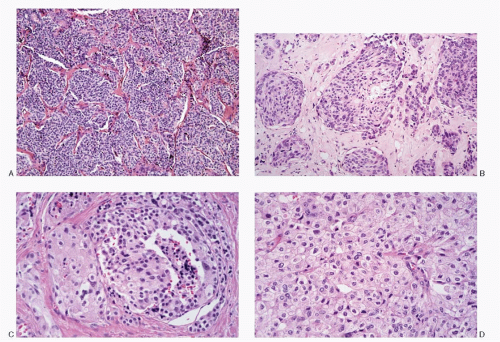
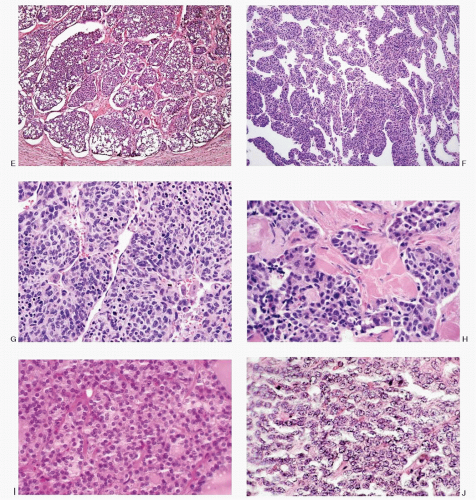
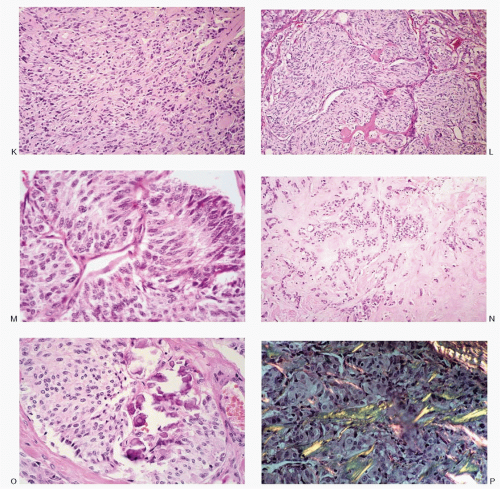
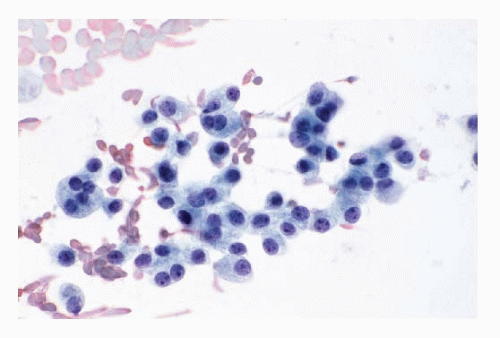
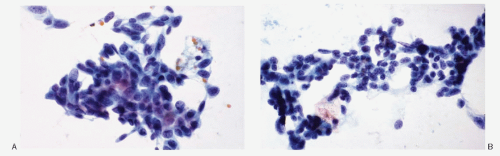
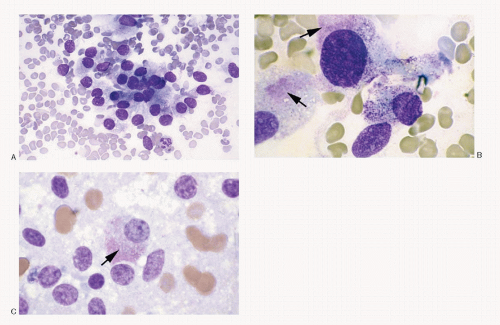
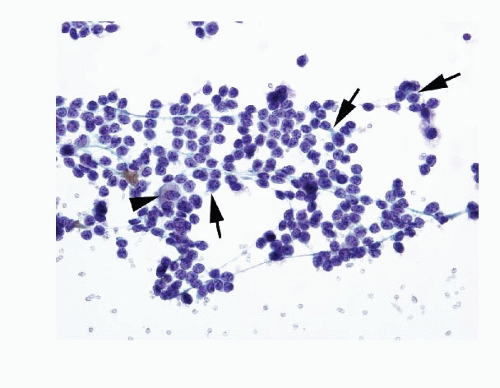
Fig. 8.3A-O) are similar in both the familial type and the sporadic type showing multiple growth patterns such as lobular, insular, trabecular, solid, rarely follicular or papillary, and fascicular, the latter formed by the spindle cells simulating a sarcomatous pattern. The nests of tumor cells vary in size, separated by variable stromal tissue, imparting an organoid pattern. The medullary carcinoma cell types vary in size and shapes, ranging from small, medium, to large with occasional giant forms. Their shapes vary from round to oval, carcinoid-like cells, cuboidal, plasmacytoid, polygonal or epithelioid, to spindle shape, triangular, and caudate to racket shaped. Their cell borders are well to poorly defined, and the cytoplasm varies from scant to abundant, clear, pale, to dense. Some may contain
mucin. Their nuclei can be monomorphic to pleomorphic, round, oval, oblong to spindle shape with stippled to coarsely granular chromatin popularly referred to as salt-pepper chromatin. Intranuclear cytoplasmic inclusions are almost always present. Hemorrhage, necrosis, and mitotic activity are infrequent findings in medullary carcinomas. A given tumor may be monomorphic showing a predominant cell type or pleomorphic with mixed patterns containing several cell types. The latter is more frequent.
Spectrum of Histopathologic Patterns of Medullary Carcinoma
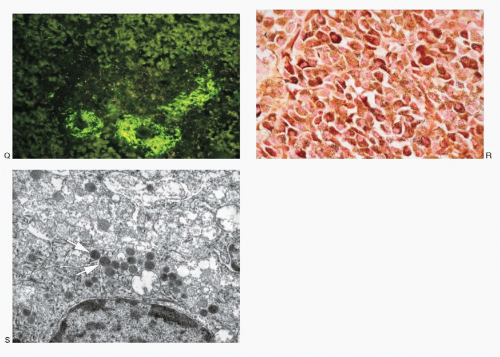
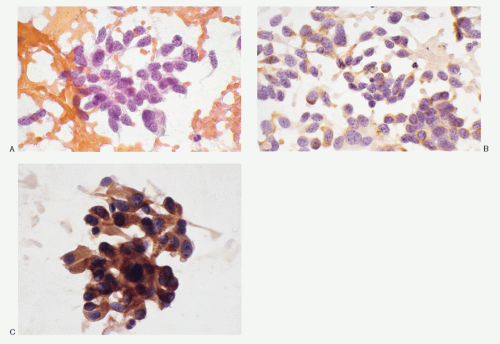
Medullary carcinoma is characterized by the presence of amyloid deposits in the stroma in up to 80% of the cases (
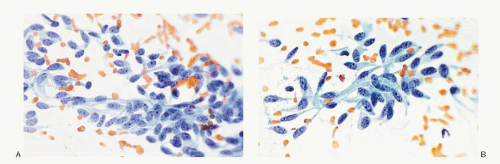
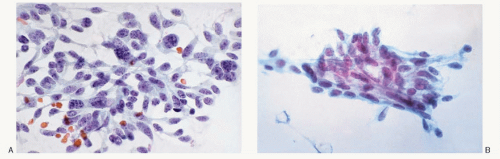
Fig. 8.3H,N). The amyloid shows green birefringence under crossed polarized light when stained by Congo red (
Fig. 8.3P) and green fluorescence under ultraviolet light when stained by thioflavin T (
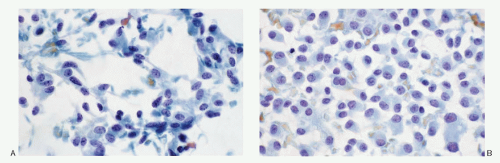
Fig. 8.3Q). Medullary carcinoma cells react strongly to calcitonin antibody (
Fig. 8.3R).
The medullary carcinoma may also contain a significant amount of collagenous stroma, with (
Fig. 8.3O) or without calcification. Several morphologic variants of medullary carcinoma have been described. These include follicular or tubular, papillary, small cell, giant cell, clear cell, melanotic (pigmented), oncocytic, squamous, amphicrine (composite calcitonin and mucin-producing), and paraganglioma-like.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access