Pancreatic neuroendocrine neoplasms, previously referred to as pancreatic endocrine tumors (PETs), are uncommon, constituting 1% to 2% of all pancreatic neoplasms. In the United States, their incidence is estimated at <1 per 100,000 people. Pancreatic neuroendocrine neoplasms were previously referred to as “islet cell tumor/islet cell carcinoma,” because of the assumption that they arise from the islets of Langerhans. Pancreatic endocrine neoplasms are now considered to be arising from the multi-potential epithelial cells in pancreatic ductules.
ENDOCRINE PANCREAS
HISTOLOGY AND CYTOLOGY
Majority of the pancreas (85%) consists of exocrine component that includes acinar tissue and ductal system (
Fig. 5.1) and the remaining represents the endocrine tissue. Ninety percent of all pancreatic endocrine cells in the adult pancreas reside in the islets of Langerhans (
Fig. 5.1 A). Fewer than 10% are distributed singly or in clusters among the ductal cells or paraductular acinar cells. The islets consist of aggregates of lightly staining endocrine cells, well demarcated from the surrounding exocrine tissue. They are embedded in reticulin and collagen fibers and lack a true capsule. Two types of islets are recognized based on their architecture and location: ordinary or regular islets and pancreatic polypeptide (PP)-rich irregular islets. The regular islets are evenly distributed in the anterior part of the head, body, and tail of the pancreas. They are round to ovoid, arranged into ribbons or lobules. The diameter of the ordinary islets ranges from 75 to 225
µm. The PP-rich irregular islets are large, 400 to 500
µm, and have trabecular architecture. The islets are penetrated by a rich network of anastomosing sinusoids. The innervation of the islets are derived from sympathetic and parasympathetic nerve fibers, closely associated with the blood vessels.
Each islet of Langerhans is composed of approximately 1,000 endocrine cells, filled with characteristic neurosecretory granules. Four major cell types are recognized in the islets, namely, alpha secreting glucagons, beta secreting insulin, delta secreting somatostatin, and PP cells secreting PP. Each islet cell produces a single peptide. Ultrastructurally, secretory granules of each type have distinctive appearance: (1) Alpha-cell granules measure 180 to 300 nm. They are round and contain an eccentric electron-dense core within a less dense peripheral region. There is a thin halo beneath the limiting membrane. These constitute 15% to 20% of total endocrine cells. (2) Beta-cell granules measure 225 to 375 nm constituting 60% to 70% of total endocrine cells. They are polymorphous and contain crystalline cores with a wide halo beneath the limiting membrane. (3) Delta-cell granules constitute 2% to 10% of total endocrine cells. The granules measure 170 to 220 nm and are round with a moderately dense core surrounded by a very thin halo. (4) The granules of PP cells constitute 15% to 20% of total endocrine cells and measure 138 to 208 nm. The granules are smaller and have homogeneous hypodense cores.
PANCREATIC NEUROENDOCRINE NEOPLASMS
Pancreatic neuroendocrine neoplasms or tumors (NETs) were classified by the World Health Organization (WHO) in 2010 (see
Appendix) on the bases of criteria similar to those for neuroendocrine neoplasms of the gastrointestinal tract (see
Chapter 4; Appendixes I and II). This classification also introduces a grading system determined by proliferative rate replacing the grade-and stage-based system as recommended in 2004 WHO classification.
PANCREATIC NEUROENDOCRINE TUMORS
Majority of the pancreatic neuroendocrine neoplasms fall in the category of pancreatic NET Grade I and Grade II, previously classified (WHO, 2004) as well-differentiated endocrine tumors, well-differentiated endocrine tumor with unknown potential, and well-differentiated carcinoma. Cytologically, these three types cannot be differentiated and are described together.
PETs can be nonfunctioning (30% to 40%) or functioning with elaboration of a number of different hormones. The relative frequency of PETs is estimated to be 30% for insulinoma, 16% to 30% for gastrinoma, 10% for vasoactive intestinal polypeptide-secreting tumor, 5% to 6% for glucagonoma, and 25% to 36% for nonfunctioning tumors.
PETs occur either sporadically or as a part of hereditary syndromes. The sporadic tumors are solitary, whereas pancreatic tumors in hereditary settings such as multiple neuroendocrine neoplasia Type I and von Hippel-Lindau disease are frequently multiple (
Table 5.1).
CLINICAL FEATURES
The mean reported age at presentation for pancreatic NET is 58 years, with a range of 12 to 78 years. The patients with hereditary syndromes are usually younger with a mean age of 35 years. There is no gender predilection. The presenting symptoms in nonfunctioning tumors are related to the size of the tumor, which remains silent for a long period of time. The presenting symptoms include abdominal pain, cachexia, weight loss,
and jaundice. In case of functioning tumors, the presenting signs depend on the effects of hormone secreted by the tumor.
Most of the pancreatic NETs follow an indolent course; they may metastasize to distant organs several years after the initial diagnosis have been made. The organs most frequently involved are liver, lymph nodes, and lungs. More than 90% of insulinomas are benign and cured by complete surgical removal.
RADIOGRAPHIC FINDINGS
Radiographically, pancreatic neuroendocrine can be detected as localized mass lesions. Magnetic resonance imaging can also detect peripancreatic lymph node and liver involvement.
GROSS AND MICROSCOPIC FEATURES
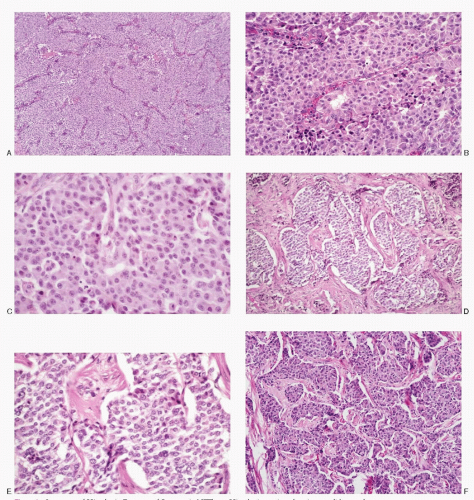
Regardless of the functioning status of the tumor and the type of hormone elaborated by the tumor, the gross findings and the cytohistomorphology remain similar in all PETs. Grossly, PETs (
Fig. 5.2) are well demarcated and well circumscribed, may or may not be encapsulated, and can occur anywhere in the pancreas but commonly involve body and the tail. Their size is variable. The functioning tumors are usually smaller than the nonfunctioning ones. The cut surface may be soft, red to yellow-tan, hemorrhagic, or grey and firm with fibrosis, or may show extensive cystic change. Larger tumors may show lobulations. Cystic change is frequent.
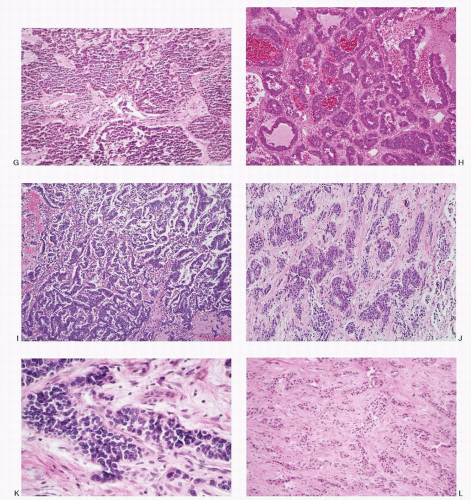
Microscopically, several architectural patterns are recognized (
Fig. 5.3): (1) trabecular (gyriform), (2) acinar, and (3) solid or diffuse. The sheets or nests of cells may be separated by strands of fibrous tissue or closely packed with a thin vascular stroma. Frequently cells are arranged around the blood vessels or spaces with eosinophilic material. The growth pattern in these tumors varies
considerably within the same tumor. Although the architectural pattern suggests the endocrine nature, there is no reliable relationship between architectural patterns and the cell type, hormone production, or biologic behavior. The tumor cells are generally uniform in size and shape. They may be round, polygonal, or elongated. The cell borders are usually well-defined. The cytoplasm is eosinophilic or clear. The nuclei are uniform, and the nucleoli range from inconspicuous to prominent. The salt-pepper chromatin is characteristic but not universally present. Pleomorphic large cells may be scattered throughout the tumor. Mitoses are generally rare. The cytoplasm is usually scant but can be appreciable and eosinophilic in oncocytic variants. PETs are richly vascular with a prominent capillary network. Hyalinization of the tumor stroma is common and may be extensive. Amyloid is sometimes present, especially in insulinomas.
HISTOCHEMISTRY
The pancreatic neuroendocrine tumor cells are both argentophilic and argyrophilic and stain positively with Fontana-Masson and Grimelius, respectively. Periodic acid-Schiff (PAS) and mucin stains are negative.
IMMUNOPROFILE
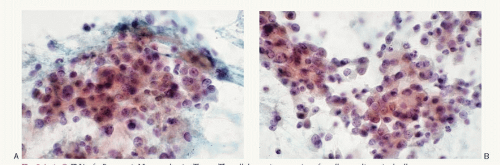
PETs react positively to cytokeratin and pan-neuroen-docrine markers such as chromogranin, synaptophysin, neuron-specific enolase, PGP 9.5, CD56, or CD57. In addition, peptide hormones can be detected by using specific antibodies. Immunohistochemical assessment of specific hormone production is valuable and correlates with the clinical symptoms and the blood profile.
ULTRASTRUCTURE
Ultrastructurally, the pancreatic NETs demonstrate dense core neurosecretory granules, ranging from 80 to 300 nm in diameter and with a tendency to cluster around Golgi complexes. Two subtypes of neurosecretory granules present a characteristic ultrastructural pattern that relates to the product synthesized. The first displays a crystalline core and an irregular submem-branous halo and correspond to insulin granules (see
Chapter 1;
Fig. 7.1B). The second shows dual density of an internal core structure, with only an indistinct halo and no crystalline configurations. This corresponds to glucagon granules.
CYTOPATHOLOGIC FEATURES
The specimens for cytopathologic diagnosis include mostly fine needle aspiration (FNA) biopsies of the pancreatic mass lesions via endoscopic ultrasound (EUS) guidance. Brushings of the biliary ducts and pancreatic ducts obtained via endoscopic retrograde cholangiopancreatography (ERCP) are also utilized. Metastatic tumors can be identified by fine needle biopsies. The deep-seated organs are sampled mostly under radiologic guidance. Body cavity fluids are also evaluated for the metastatic spread to the serous membranes (
Table 5.2).
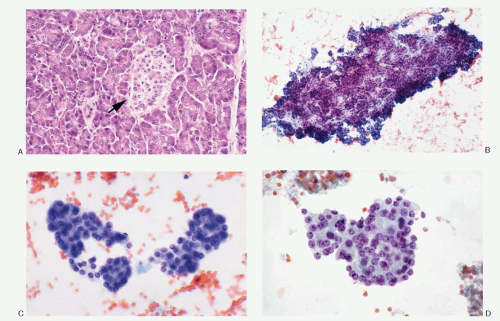
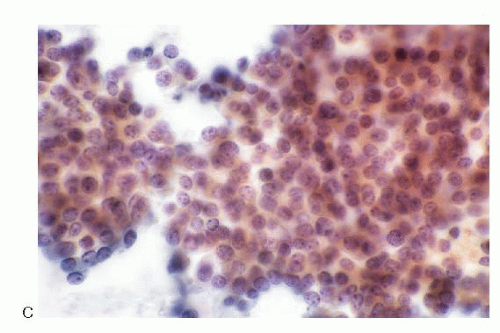
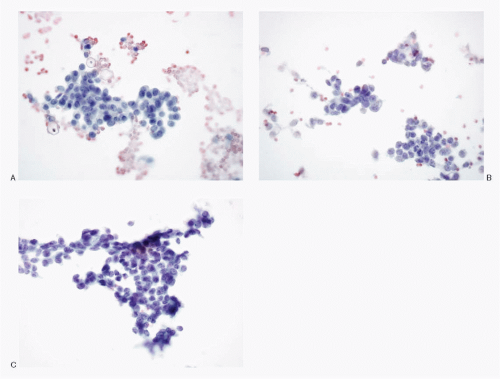
The cytologic presentation of the pancreatic NETs is characteristic (
Table 5.3;
Figs. 5.4,
5.5,
5.6,
5.7,
5.8,
5.9,
5.10,
5.11,
5.12,
5.13,
5.14,
5.15,
5.16 and
5.17). The aspirates are generally very cellular. The neoplastic cells are small to medium-sized and are either uniform and monomorphic or may demonstrate pleomorphism. They present themselves as isolated and in loosely cohesive groups forming a dispersed cell pattern (
Fig. 5.11D) or in syncytial tissue fragments. The latter may present trabecular pattern, insulae, rosettes or may form no architectural configuration. The neoplastic cells are small to medium-sized, either round, cuboidal to plasmacytoid, and very rarely spindle shaped (
Fig. 5.15) with central or eccentric nuclei. Spindle and large giant forms are occasionally present. A given case of pancreatic NETs may show a monomorphic pattern
(
Figs. 5.7,
5.9,
5.10,
5.11 and
5.12, and
5.14) or a pleomorphic pattern (
Figs. 5.4,
5.5 and
5.6,
5.8,
5.9, and
5.13). The nuclei are round to oval; nuclear membranes are smooth and crisp. The chromatin is characteristic of the neuroendocrine family of tumors, being uniformly and coarsely granular presenting a salt-pepper pattern. Nucleoli are not consistently present. The nuclei tend to be uniform but may be variable in size. The cytoplasm ranges from scant to moderate, pale to dense, and is eosinophilic in oncocytic variant. Capillary network may be prominent in the background and neoplastic cells may be seen in perivascular location. Mitotic activity or necrosis is usually not present but can be seen in widely invasive NETs (
Fig. 5.5). The cell block preparations can be extremely useful in the diagnostic evaluation since they allow ancillary testings.




 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access