This chapter covers neuroendocrine neoplasms of the genitourinary tracts, which occur only infrequently. Neuroendocrine neoplasms are more common in genital tracts and more common in the female than in the male genital tract as compared to the urinary tract. In females, the uterine cervix is the frequent site, followed by ovaries. Neuroendocrine tumors of the endometrium, vagina, and vulva also have been reported in the literature.
Male genital tract neuroendocrine tumors are of unusual occurrence. However, they involve prostate more frequently than the rest of the organs. Other neuroendocrine tumors include testicular carcinoids and small cell neuroendocrine carcinomas that occur rarely in the scrotum, penis, and penile urethra. Urinary tract neuroendocrine neoplasms are very uncommon.
The cytologic diagnosis of neuroendocrine tumors of the genitourinary tract is provided only from cervical smears in cases of poorly differentiated neuroendocrine carcinomas of the uterine cervix. Rarely small cell carcinomas of the urinary bladder may exfoliate in the urine and identified via cytologic examination. Except for these two sites, a primary cytologic diagnosis of genitourinary tract neuroendocrine tumors is not generally made. Most of these neoplasms are identified by either surgical biopsies or excision. Metastatic sites such as lymph nodes, liver, or lungs are occasionally subjected to fine needle biopsy procedures for the cytologic diagnosis. Body cavity fluids may also be examined for the cytologic diagnosis. Overall, the documentation of cytologic features, therefore, is very lean.
This chapter covers neuroendocrine neoplasms of the female genital tract, followed by male genital tract, prostate, and urinary tract.
NEUROENDOCRINE TUMORS OF THE FEMALEGENITAL TRACT
Neuroendocrine tumors of the female genital tract, although very rare, have been described in various sites such as endometrium, cervix, fallopian tubes, vagina, vulva, and ovaries. Of these, small cell carcinoma (poorly differentiated neuroendocrine carcinoma) of the uterine cervix and carcinoid tumors of the ovaries are more frequent and will be described in detail.
POORLY DIFFERENTIATED NEUROENDOCRINE (SMALL CELL) CARCINOMA OF THE UTERINE CERVIX
Small cell neuroendocrine carcinoma is a highly aggressive malignant neoplasm of the uterine cervix, accounting for 3% to 5% of all cervical cancers. The incidence is 0.5/100,000 in the United States. There is no racial predilection. Patient’s average age is 36 to 42 years, and the patients are typically younger than those with squamous carcinomas. Up to 80% of small cell carcinomas are associated with human papilloma virus (HPV), type 18. HPV type 16 has been detected in a minority of patients with small cell carcinomas.
The patients may be asymptomatic or clinically present with vaginal bleeding. Less frequently, they may present with paraneoplastic syndromes such as Cushing syndrome, carcinoid syndrome, and hypoglycemia. Small cell carcinomas are very aggressive tumors and metastasize widely. Greater than 75% of the patients are dead from the disease within 1 year. The tumors are detected less often on screening cervical smears than squamous cell carcinomas.
GROSS AND HISTOLOGIC FEATURES
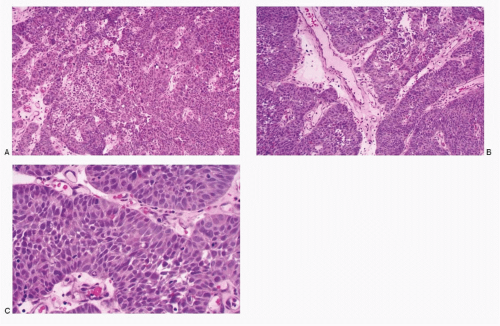
The smaller lesions are inconspicuous on gross examination of the cervix. Larger lesions may present as ulcerated masses, often invading the deeper structures. Microscopic examination reveals a pattern similar to its pulmonary counterpart, characterized by large islands and masses of small, round, oval-to-short spindle cells with scant indiscernible cytoplasm and large nuclei and very high N/C ratios (
Fig. 12.1; see
Fig. 12.6). Palisading of the neoplastic cells at the periphery of the tumor islands is characteristic. The carcinoma also presents a trabecular pattern or a solid growth pattern. The nuclei demonstrate molding and are intensely hyperchromatic with a brisk mitotic activity. Karyorrhexis and large areas of tumor necrosis are frequent.
CYTOPATHOLOGIC FEATURES
The specimens for cytologic diagnosis mostly represent cervical/vaginal smears and occasionally aspiration biopsies of the metastatic sites or body cavity fluids. The cytopathologic features are similar to those seen in pulmonary small cell carcinomas (ref.
Chapter 3;
Table 3.7). The conventional cervical smears (
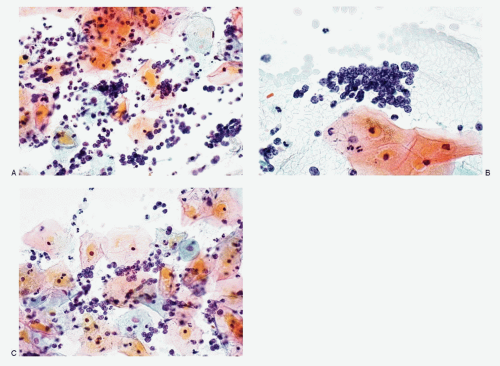
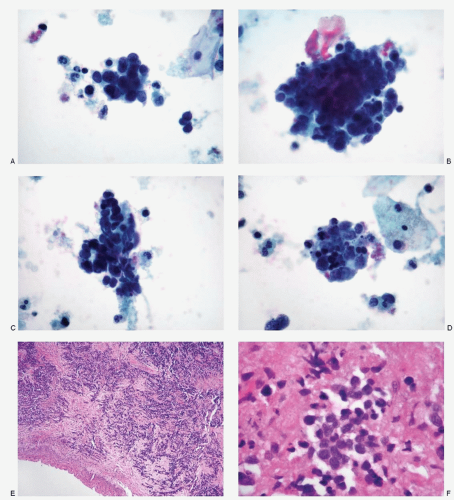
Figs. 12.2 and
12.3) present excellent cytomorphology as compared to the liquid-based preparations (
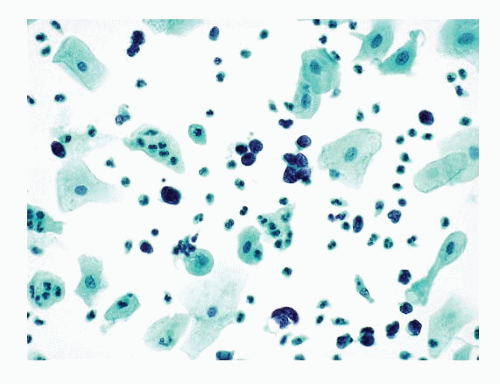
Figs. 12.4,
12.5 and
12.6). The conventional smears show varying numbers of malignant cells, isolated, in loosely cohesive groups and in syncytial tissue fragments without any architectural patterns. The neoplastic cells are small with high N/C ratios. Their nuclei are round to oval, occasionally oblong to short spindle shaped, containing coarsely granular chromatin with either a salt-pepper appearance (
Figs. 12.2 and
12.3) or deep-staining compact pattern. Nucleoli are not conspicuous, and nuclear molding is often noted. In the liquid-based preparations, the small cell carcinoma cells tend to aggregate in three-dimensional balls or present in a dispersed fashion (
Figs. 12.4,
12.5 and
12.6). These dispersed single cells can easily be overlooked or mistaken for endometrial cells. Within these three-dimensional balls, the nuclei of the small cell carcinoma cells in liquid-based
preparations are extremely crowded and overlapped and their morphology may not be well visualized.
IMMUNOPROFILE
The small cell carcinoma cells are immunoreactive to neuroendocrine markers such as chromogranin, synaptophysin, neuron-specific enolase, TTF-1, CD56, and cytokeratin.
ULTRASTRUCTURE
Ultrastructurally, cells of small cell carcinomas demonstrate dense-core neurosecretory granules.
DIAGNOSTIC DIFFICULTIES AND DIFFERENTIAL DIAGNOSES
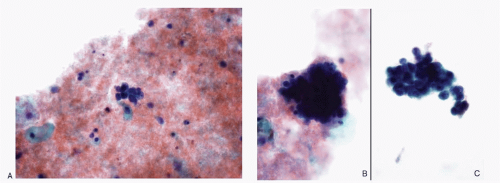
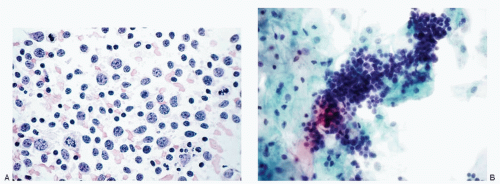
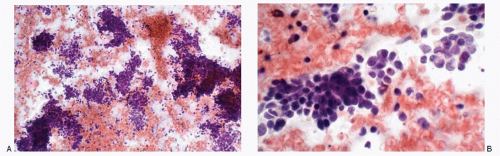
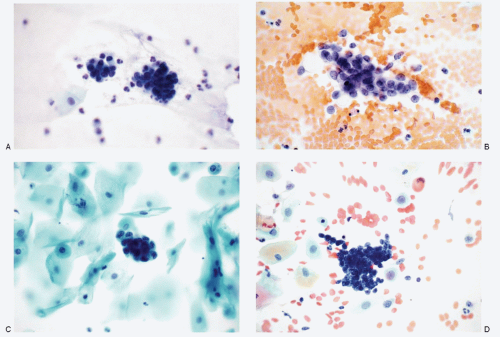
Recognition of malignant cells originating from small cell neuroendocrine carcinoma may be compromised because of small number of cells, poor cytologic details, or misinterpretation. The differential diagnoses of small cell carcinomas of the cervix include diagnostic entities that are composed of cells with a small size, namely endometrial cells, endometrial adenocarcinoma, high-grade squamous intraepithelial lesion, and poorly differentiated squamous carcinoma, florid follicular cervicitis, and malignant lymphoma. The differential diagnostic features are listed in
Table 12.1 and illustrated in
Figures 12.7,
12.8,
12.9,
12.10,
12.11,
12.12 and
12.13.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access