Anatomically, the mediastinum is defined as a space between the two pleural cavities, with its boundaries formed superiorly by the thoracic inlet, inferiorly by the diaphragm, anteriorly by the sternum, and posteriorly by the spinal column. The mediastinum is subdivided arbitrarily into superior and inferior compartments by an imaginary line extending from the manubrium sterni to the fourth thoracic vertebrae. The inferior mediastinum again is subdivided into anterior, middle, and posterior compartments. Besides the major anatomic structures such as pericardial cavity containing the heart, major blood vessels, trachea, major bronchi, and esophagus, the mediastinum contains thymus, lymph nodes, soft tissues, nerve trunks, and paraganglia.
Neuroendocrine tumors of the mediastinum include those derived from neuroendocrine cells within the thymus (anterior mediastinum), from paraganglionic rests, or from misplaced embryonal structures within the mediastinum such as parathyroid tissue. Also encountered are primitive neuroectodermal tumors and neuroblastomas. This chapter covers mainly thymic neuroendocrine tumors since they are the most common neuroendocrine neoplasms in the mediastinum.
PRIMARY NEUROENDOCRINE CARCINOMAS OF THE THYMUS
Primary neuroendocrine carcinomas (NECs) of the thymus are uncommon tumors accounting for 2% to 4% of all anterior mediastinal neoplasms. They include carcinoid tumors, atypical carcinoids, small cell carcinoma, and large cell NEC. Suster and Moran recommended a three-tier classification for thymic endocrine tumors, namely, low-grade or well-differentiated NEC (typical carcinoid tumors), intermediate-grade or moderately differentiated NEC (atypical carcinoid tumors), high-grade poorly differentiated NEC small cell carcinomas, and large cell NECs (
Table 6.1). World Health Organization (WHO) classification for thymic neuroendocrine tumors (Appendix) includes two categories, namely, well differentiated for carcinoid tumors (typical and atypical) and poorly differentiated for small cell carcinoma and large cell NECs. Both terminologies are used in this chapter.
Majority of the thymic NEC are either well differentiated (typical carcinoid tumor) or moderately differentiated (atypical carcinoid). The poorly differentiated NEC of thymus are very uncommon.
ASSOCIATION WITH HEREDITARY SYNDROMES
Thymic NECs may be associated with multiple neuroendocrine neoplasia syndrome type I (MEN I).
WELL AND MODERATELY DIFFERENTIATED NEUROENDOCRINE CARCINOMAS (TYPICAL AND ATYPICAL CARCINOID TUMORS) OF THE THYMUS
Thymic carcinoid tumors constitute 2% to 5% of thymic epithelial tumors. In contrast to pulmonary carcinoid tumors (well-differentiated NEC), majority of the thymic carcinoid tumors are represented by atypical carcinoid tumors (NEC, moderately differentiated, or intermediategrade) and are noted to follow a more aggressive behavior than its counterparts in other locations such as lungs. According to published reports, 80% of thymic carcinoid tumors exhibited malignant behavior as compared to 26% of bronchial carcinoid tumors.
CLINICAL FEATURES
The thymic NECs occur in all age groups, but show predilection for adults between 40 and 60 years of age, with a male:female ratio of 3:1. About 50% of well-differentiated NECs exhibit local symptoms such as chest pain, cough, and dyspnea. Acute symptoms such as superior venacaval syndrome are uncommon. Carcinoid syndrome is extremely rare. However, up to 30% of adults and over 50% of children with thymic carcinoid tumors are associated with Cushing syndrome due to ectopic adrenocorticotropic hormone production. About 25% of thymic carcinoids have a positive family history of MEN 1 syndrome (see
Chapter 1,
Table 1.1). Thymic NECs follow an aggressive course. Fifty percent show local metastasis, invasion into adjacent organs, and pleural or pericardial cavity. Lymph node metastases include mediastinal, cervical, and supracervical lymph nodes. Hematogenous spread to distant organs has been reported.
GROSS AND HISTOLOGIC FEATURES
Thymic carcinoid tumors are unencapsulated. Grossly, they are either well defined or invasive. Their size ranges from 2 to 20 cm with a mean of 8 to 10 cm. Their cut surface is gray-white and gritty. The consistence is firm. They usually lack the lobulated pattern of thymomas. Oncocytic variant may show tan or brown discoloration. Foci of hemorrhage and necrosis are present in 70% of the cases. Calcifications are frequent.
Histologically, thymic carcinoids exhibit a wide spectrum of patterns that depends on the degree of differentiation and biologic behavior. Some demonstrate the histologic pattern of a pulmonary typical carcinoid tumor (see
Fig. 3.2A-G), with mild cytologic atypia, scattered mitotic figures (<3 per 10 high power field [HPF]), and small foci of necrosis. Majority exhibit pronounced cytologic atypia, increased mitotic activity (3 to 10 mitotic figures per 10 HPF), extensive areas of necrosis, and foci of vascular invasion. Majority of the NECs of the thymus show histologic patterns consistent with atypical carcinoid tumor (
Table 6.2).
Several morphologic variants of thymic endocrine carcinomas have been described. These include spindle cell type, oncocytic type, mucinous type, pigmented type, angiomatoid type, and with amyloid-like stroma.
CYTOPATHOLOGIC FEATURES
The specimens for cytopathologic diagnosis represent fine needle aspiration (FNA) biopsies performed either via percutaneous transthoracic route or transbronchial route via endoscopic ultrasound guidance. In general, fine needle biopsy of thymic lesions is not a very popular diagnostic technique and is not widely practiced. The cytologic documentation of thymic neuroendocrine tumors therefore is sparse.
The cytologic features of NECs parallel those of pulmonary carcinoid tumors (See
Chapter 3;
Tables 3.1 and
3.4) (
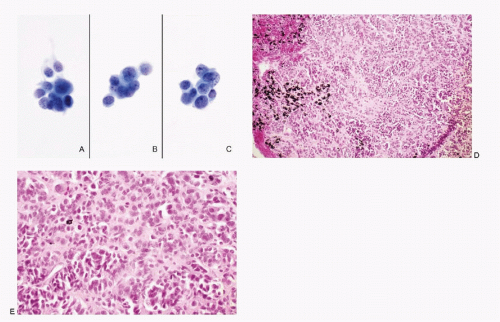
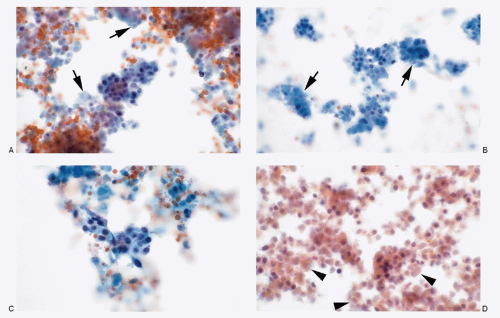
Figs. 6.1,
6.2,
6.3 and
6.4). The cellularity of the aspirate is usually variable depending on the amount of stroma and extent of necrosis. In low-grade tumors (typical carcinoid), the neoplastic cells are small, round to oval, and present as discrete or loosely cohesive cells and sometimes in syncytial tissue fragments. Their cell borders are well to poorly defined. The cytoplasm is usually scant. The nuclei are round with high N/C ratios and contain typical salt-pepper chromatin (
Fig. 6.1). The aspirates of atypical carcinoid tumors (moderately differentiated NEC) are cellular with considerable cellular and nuclear pleomorphism (
Fig. 6.3). Foci of necrosis are frequent (
Fig. 6.2). However, nuclear molding and stretch artifacts are not present.
HISTOCHEMISTRY
The cells of thymic NECs are argyrophilic, and stain with Grimelius.
IMMUNOPROFILE
Thymic carcinoid tumor cells react positively to all neuroendocrine markers, cytokeratins (CAM 5.2, AE1/AE3), and CD56 and CD57.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access