General Considerations
Nearly every infant is born with a serum bilirubin level higher than that of the normal adult. Approximately 60% of newborns are visibly jaundiced during the first week of life. The diagnostic and therapeutic challenge for the physician is to differentiate normal physiologic jaundice from pathologic jaundice, and to institute appropriate evaluation and therapy when necessary.
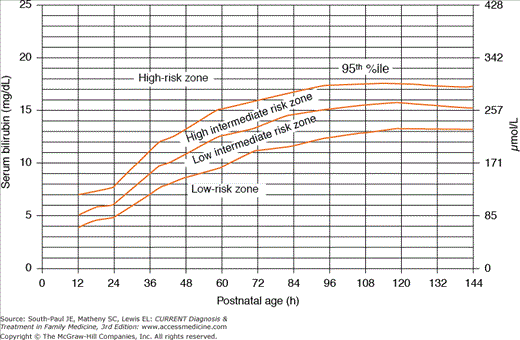
Table 3-1 lists several maternal and neonatal factors that increase the risk of developing severe hyperbilirubinemia among infants of 35 or more weeks’ gestation. Among the most significant clinical characteristics associated with severe hyperbilirubinemia are predischarge levels in the high-risk zone on the serum bilirubin nomogram (Figure 3-1). The following factors (in order of decreasing importance) are associated with decreased risk of significant jaundice: total serum bilirubin (TSB) or transcutaneous bilirubin (TcB) level in the low-risk zone, gestational age greater than 41 weeks, exclusive bottle-feeding, black race, and discharge from the hospital after 72 hours.
Figure 3-1.
Nomogram for designation of risk in 2840 well newborns of 36 or more weeks’ gestational age with birth weight of 2000 g or more or 35 or more weeks’ gestational age and birth weight of 2500 g or more based on the hour-specific serum bilirubin value. (Reproduced, with permission, from American Academy of Pediatrics Subcommittee on Hyperbilirubinemia: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297.)
| Major Risk Factors |
| Predischarge TSB or TcB level in the high-risk zone (see Figure 3-1) |
| Jaundice observed in the first 24 h of life |
| Blood group incompatibility with positive direct antiglobulin test, other known hemolytic disease (eg, G6PD deficiency), elevated ETCO |
| Gestational age of 35-36 wk |
| Previous sibling who received phototherapy |
| Cephalohematoma or significant bruising |
| Exclusive breastfeeding, particularly if nursing is not going well and weight loss is excessive |
| East Asian race |
| Minor Risk Factors |
| Predischarge TSB or TcB level in the high intermediate-risk zone |
| Gestational age of 37-38 wk |
| Jaundice observed before discharge |
| Previous sibling with jaundice |
| Macrosomic infant of diabetic mother |
| Maternal age >25 y |
| Male gender |
Pathogenesis
The three classifications of neonatal hyperbilirubinemia are based on the following mechanisms of accumulation: increased bilirubin load, decreased bilirubin conjugation, and impaired bilirubin excretion. In the newborn, unconjugated bilirubin is produced faster and removed more slowly than in the normal adult due to immaturity of the glucuronyl transferase enzyme system. The main source of unconjugated bilirubin is the breakdown of hemoglobin in senescent red blood cells. Newborns have an increased erythrocyte mass at birth (average hematocrit of 50% vs 33% in the adult) and a shorter life span for erythrocytes (90 days vs 120 days in the adult). The newborn cannot readily excrete unconjugated bilirubin, and much of it is reabsorbed by the intestine and returned to the enterohepatic circulation.
Increased production and decreased elimination of bilirubin lead to a physiologic jaundice in most normal newborns. Bilirubin is a very effective and potent antioxidant, and physiologic jaundice may provide a mechanism for protecting the newborn from oxygen free-radical injury. The average full-term white newborn experiences a peak serum bilirubin concentration of 5-6 mg/dL (86-103 μmol/L), which begins to rise after the first day of life, peaks on the third day of life, and falls to normal adult levels by days 10-12. African American infants tend to have slightly lower peaks in serum bilirubin. In Asian infants, serum bilirubin levels rise more quickly than in white infants and tend to reach higher peaks on average (8-12 mg/dL; 135-205 μmol/L). This leads to a longer period of physiologic jaundice among Asian and Native American newborns. Preterm infants (<37 weeks’ gestation) of all races may take 4-5 days to reach peak serum bilirubin levels, and these peaks may be twice that observed among full-term infants.
Infants who are breast-fed may experience exaggerated bilirubin levels due to two separate phenomena associated with breastfeeding and breast milk.
Breast-fed infants may experience relative starvation in the first few days of life due to delayed release of milk by the mother or difficulties with breastfeeding. This nutritional inadequacy can result in increased enterohepatic circulation of bilirubin, leading to elevated serum bilirubin levels in the first few days of life. Termed breastfeeding jaundice, this finding is considered abnormal and can be overcome by offering frequent feedings (10-12 times per day) and by avoiding water supplementation in breast-fed infants.
Breast milk is believed to increase the enterohepatic circulation of bilirubin; however, the specific factor(s) in breast milk that are responsible for this action are unknown. For the first 5 days of life, the serum bilirubin level in breast-fed infants parallels that in non–breast-fed infants. Beginning at approximately day 6, breast milk jaundice occurs in breast-fed infants as serum bilirubin either rises a little for a few days or declines more slowly. Approximately two-thirds of breast-fed infants may be expected to have hyperbilirubinemia from 3 weeks to 3 months of age, with as many as one-third exhibiting clinical jaundice. Breast milk jaundice (unlike breastfeeding jaundice) is considered a form of normal physiologic jaundice in healthy, thriving breast-fed infants.
Exaggerated physiologic jaundice occurs at serum bilirubin levels between 7 and 17 mg/dL (104-291 μmol/L). Bilirubin levels above 17 mg/dL in full-term infants are no longer considered physiologic, and further investigation is warranted.
The onset of jaundice within the first 24 hours of life or a rate of increase in serum bilirubin exceeding 0.5 mg/dL/h (8 μmol/L/h) is potentially pathologic and suggestive of hemolytic disease. Conjugated serum bilirubin concentrations exceeding 10% of total bilirubin or 2 mg/dL (35 μmol/L) are also not physiologic and suggest hepatobiliary disease or a general metabolic disorder.
Table 3-2 summarizes factors that may indicate that jaundice is pathologic as opposed to physiologic, warranting further evaluation. Important historical features include family history of hemolytic disease, onset of jaundice in the first 24 hours of life, a rapid rise in serum bilirubin levels, and ethnicity, as well as infant feeding patterns, stool and urine appearance, and activity levels. Clinical assessment requires careful attention to vital signs, weight loss, general appearance, pallor, and hepatosplenomegaly.
| General considerations | Family history of significant hemolytic disease |
| Onset of jaundice before age of 24 h | |
| Rise in serum bilirubin levels of more than 0.5 mg/dL/h | |
| Pallor, hepatosplenomegaly | |
| Rapid increase in TSB level after 24-48 h (consider G6PD deficiency) | |
| Ethnicity suggestive of inherited disease (G6PD deficiency, etc) | |
| Failure of phototherapy to lower TSB level | |
| Clinical signs suggesting possibility of other diseases (eg, sepsis, galactosemia) in which jaundice may be one manifestation | Vomiting |
| Lethargy | |
| Poor feeding | |
| Hepatosplenomegaly | |
| Excessive weight loss | |
| Apnea | |
| Temperature instability | |
| Tachypnea | |
| Signs of cholestatic jaundice suggesting the need to rule out biliary atresia or other causes of cholestasis | Dark urine or urine positive for bilirubin |
| Light-colored stools | |
| Persistent jaundice of more than 3 weeks’ duration |
The primary concern with severe hyperbilirubinemia is the potential for neurotoxic effects as well as general cellular injury, which can occur at TSB levels exceeding 20-25 mg/dL. The term kernicterus refers to the yellow staining of the basal ganglia observed postmortem among infants who died with severe jaundice. (Bilirubin deposition in the basal ganglia can also be imaged using magnetic resonance techniques.) The American Academy of Pediatrics (AAP) has recommended that the term acute bilirubin encephalopathy be used to describe the acute manifestations of bilirubin toxicity seen in the first weeks after birth and that the term kernicterus be reserved for the chronic and permanent clinical sequelae of bilirubin toxicity.
Although a common complication of hyperbilirubinemia in the 1940s and 1950s due to Rh erythroblastosis fetalis and ABO hemolytic disease, kernicterus is rare today with the use of Rh immunoglobulin and with the intervention of phototherapy and exchange transfusion. With early discharge to home, however, a small resurgence of kernicterus has been observed in countries in which this complication had essentially disappeared. For instance, although no cases of kernicterus were identified in Denmark during the 20 years preceding 1994, six cases were diagnosed between 1994 and 1998. Although a few isolated cases of kernicterus have been reported in the United States in the last two decades, no published data on the incidence or prevalence of kernicterus in the United States are available.
Bilirubin can interfere with DNA synthesis as well as protein synthesis and protein phosphorylation. Bilirubin can also interfere with neuroexcitatory signals and impair nerve conduction, particularly in the auditory nerve. Hyperbilirubinemia may also impair cerebral glucose metabolism in the brain.
The concentration of bilirubin in the brain and the duration of exposure are important determinants of the neurotoxic effects of bilirubin. Bilirubin can enter the brain when not bound to albumin, so infants with low albumin are at increased risk of developing kernicterus. Conditions that alter the blood-brain barrier such as infection, acidosis, hypoxia, sepsis, prematurity, and hyperosmolarity may affect the entry of bilirubin into the brain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree