Neonatal Enterocolitis and Short Bowel Syndrome
Brad W. Warner
Jacqueline M. Saito
Despite dramatic progress in the overall care of the premature infant, neonatal necrotizing enterocolitis (NEC) remains a significant cause of morbidity and mortality. The underlying cause of NEC has not yet been identified and is likely multifactorial. The pathophysiology of NEC and strategies to prevent development of NEC continue to be actively investigated. For infants who require surgical intervention, research efforts have focused on improving patient survival, minimizing intestinal loss, and assessing neurodevelopmental outcomes. Infants who require massive intestinal resection are at high risk for developing short bowel syndrome (SBS) and long-term dependence on parenteral nutrition (PN). SBS is characterized by insufficient intestinal absorptive capacity to sustain normal growth and development. The underlying cause of SBS may be acquired, as with NEC, or congenital. In conjunction with medical management, surgical intervention has an important role in the prevention and treatment of SBS complications.
Neonatal Necrotizing Enterocolitis
NEC is an acquired condition in which the intestine develops acute inflammation. The inflammation may affect the small intestine and/or colon in a focal, patchy, or extensive distribution, and may progress to necrosis and perforation. Risk factors for NEC include low birth weight and prematurity. Though NEC may affect full-term infants, approximately 90% of cases occur in infants born under 36 weeks’ gestation. The estimated incidence of NEC ranges from 0.5 to 5 per 1,000 live births. Up to 7% of very low birth weight (VLBW) infants (birth weight <1,500 g) may develop NEC. Case fatality rates for NEC range from 12% to 30% and are higher among infants who require surgical intervention.
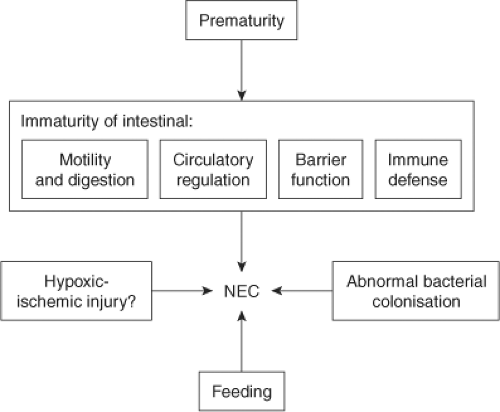 Fig. 1. Pathophysiology of NEC. Reprinted from The Lancet, 368, Lin PW, Stoll BJ, Necrotising enterocolitis, 1271–83, 2006, with permission from Elsevier. |
The pathophysiology of NEC has been the subject of intense investigation but remains incompletely understood. In retrospective studies of NEC, the role of feeding practices (breast milk vs. formula, rate at which feedings are increased) has been examined. An infectious etiology has been suggested by clusters of NEC cases within neonatal intensive care units. Hypotension and hypoxia have been less strongly associated with NEC; however, comorbidities such as congenital heart disease, perinatal asphyxia, and respiratory distress are often present in term neonates who develop NEC. Experimental studies have explored the role of intestinal immaturity, abnormal intestinal microbe colonization, and immature regulation of intestinal circulation (Fig. 1). Features of the premature intestine that may confer susceptibility to NEC are abnormal or immature bowel motility, digestive capacity, epithelial barrier function, and immune function.
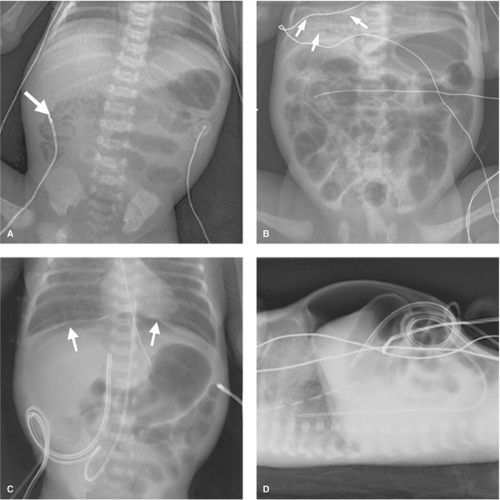
The clinical presentation of NEC varies in severity and rapidity of onset. Bell criteria for NEC categorize the signs and symptoms into suspected, definite, and severe disease (Table 1). Infants with NEC may have a combination of symptoms such as hypotension, temperature instability, feeding intolerance with emesis or increased gastric residuals, and bloody stool. Manifestations of NEC on physical examination are mottled skin, reflecting poor peripheral perfusion, abdominal distension, abdominal discoloration, and abdominal wall erythema and edema. Because of the thin abdominal wall in premature infants, dilated, edematous loops of intestine may be visible; leaked stool from intestinal perforation may cause a blue discoloration to the abdomen. Laboratory abnormalities are generally nonspecific and resemble those seen with neonatal sepsis, such as thrombocytopenia, leukocytosis or leukopenia, elevated C-reactive protein, metabolic acidosis, and respiratory acidosis, especially if ventilation is impaired by abdominal distension. The diagnosis of NEC is usually established with plain abdominal radiographs. Pneumatosis intestinalis, or air within the intestinal wall, and portal venous gas can be transient radiographic findings (Fig. 2A,B); therefore, intermittent radiographs are typically obtained when NEC is suspected. Other radiographic signs of NEC are persistent dilation of a specific bowel loop (“fixed loop”), and separation of bowel loops due to bowel wall edema. Free intraperitoneal air may be subtle on supine abdominal radiograph and can create a lucency over the liver (Fig. 2C), with a central longitudinal line from air adjacent to the falciform ligament; free air is typically more easily seen on left lateral decubitus or cross-table lateral views (Fig. 2D). Abdominal ultrasound may reveal complex ascites in the context of intestinal perforation, evidence of gas within the portal vessels, and bowel wall thickening.
Table 1 Bell Stages of Necrotizing Enterocolitis | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Treatment of suspected and definite NEC includes bowel rest, nasogastric or orogastric decompression, broad-spectrum intravenous antibiotics, and resuscitation with intravenous fluids (Fig. 3). Pressor and ventilatory support is instituted as required by the infant’s overall status. Unfortunately, premature infants who have respiratory distress syndrome often require abrupt escalation of ventilatory support with the onset of NEC. The optimal duration of bowel rest and antibiotics for treatment of NEC is not known. For infants who recover from NEC with medical treatment, the role of contrast studies of the intestine prior to feeding is also not well established.
The clearest indication for surgical intervention in NEC is evidence of intestinal perforation, typically diagnosed with plain abdominal radiograph showing free intraperitoneal air. Relative indications are progressive clinical deterioration, as reflected by worsening hypotension, metabolic acidosis, respiratory acidosis related to abdominal distension, or thrombocytopenia, and evidence of bowel obstruction or necrosis. On physical examination, patients may have focal abdominal wall erythema or abdominal wall discoloration indicative of segmental bowel necrosis or loculated intestinal perforation. Fixed or persistent intestinal loop on plain abdominal radiographs may be indicative of bowel compromise.
Consideration of patient comorbidities and stability is essential in the preoperative preparation of the infant with NEC. Even in the absence of NEC, the respiratory status of the premature neonate is often tenuous and can rapidly deteriorate. Increased oxygen and ventilatory requirements sometimes precede the clinical recognition of NEC.
Tenuous respiratory status may render transport of a neonate to the operating room too risky and necessitate a bedside operation. In the face of massive intraperitoneal air, temporary decompression of the abdomen with a large-bore angiocatheter can significantly improve ventilation. Intravenous fluid resuscitation must be carefully administered, especially if a patent ductus arteriosus is present; overaggressive intravenous fluid administration may lead to worsened hypoxia, because of increased shunting through the pulmonary vascular bed. Anemia, thrombocytopenia, and coagulopathy necessitate the availability of appropriate blood products.
Tenuous respiratory status may render transport of a neonate to the operating room too risky and necessitate a bedside operation. In the face of massive intraperitoneal air, temporary decompression of the abdomen with a large-bore angiocatheter can significantly improve ventilation. Intravenous fluid resuscitation must be carefully administered, especially if a patent ductus arteriosus is present; overaggressive intravenous fluid administration may lead to worsened hypoxia, because of increased shunting through the pulmonary vascular bed. Anemia, thrombocytopenia, and coagulopathy necessitate the availability of appropriate blood products.
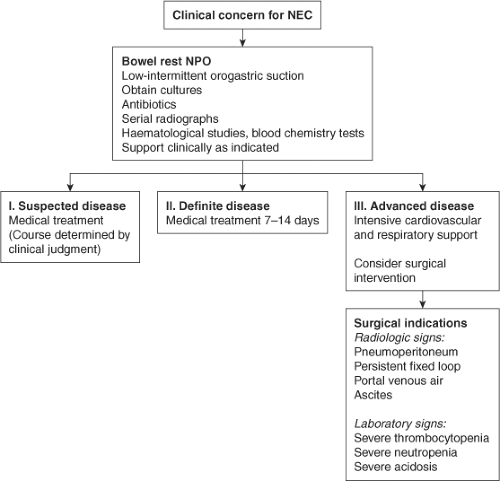 Fig. 3. Neonatal necrotizing enterocolitis (NEC) management algorithm. Reprinted from The Lancet, 368, Lin PW, Stoll BJ, Necrotising enterocolitis, 1271–83, 2006, with permission from Elsevier. |
Often influenced by the gestational age, size, and stability of a patient, the choice of surgical procedure has been the subject of multiple studies. Laparotomy traditionally has been the mainstay for treatment of neonates with intestinal perforation. Peritoneal drainage was first described by Ein in 1977 in a series of five unstable neonates with intestinal perforation. In a follow-up report in 1980, 46% of patients survived, and 49% never required laparotomy. Over the past 30 years, mixed outcomes following peritoneal drainage or laparotomy have been reported from single institutions; meta-analysis of these studies has shown that neonates treated with peritoneal drainage tend to be smaller and more premature. A prospective cohort study in 2006 through the National Institute of Child Health and Human Development (NICHD) Neonatal Network of extremely low birth weight (ELBW) infants (401 to 1,000 g) who developed NEC or isolated intestinal perforation reported 50% overall survival to discharge and a high rate of neurodevelopmental impairment at age 18 months (49% of survivors). Infants in the peritoneal drain group tended to have lower birth weight, were more premature, and had greater use of vasopressors, more severe hypotension, and higher ventilatory requirements. In addition, several infants in the peritoneal drain group were “too ill” for laparotomy. Unadjusted mortality was higher in the peritoneal drain group; with adjustment for confounders, the difference in mortality between infants treated with peritoneal drainage and laparotomy was diminished. In an effort to minimize selection bias, randomization to peritoneal drain or laparotomy was used in NECSTEPS, a multicenter trial of infants who developed intestinal perforation and weighed less than 1,500 g at birth; both peritoneal drain and laparotomy groups had similar 90-day mortality (35%), hospital length of stay, and need for total parenteral nutrition (TPN) at 90 days.
Peritoneal drain placement may be performed rapidly at the patient’s bedside (Fig. 4). After administration of intravenous sedation, the abdomen is sterilely prepped and draped. Local anesthetic is infiltrated in the right lower abdomen. A small incision is made at a site that avoids the liver edge and bladder. The subcutaneous tissue and abdominal wall muscle are spread with a fine-tipped clamp, and the peritoneal cavity is entered bluntly as confirmed by release of air and feculent ascites. The peritoneal cavity may be lavaged with warmed saline through a large-bore angiocatheter. A quarter-inch plastic drain is advanced into the peritoneal cavity and secured to the skin. A second drain may be placed in the left lower abdomen. After drainage ceases, the drain is gradually withdrawn. Clinical deterioration, bowel obstruction, and persistent fistula are among the indications for laparotomy following drain placement; up to two-thirds of patients treated with peritoneal drainage require subsequent laparotomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree