|
Typical Clinical Features |
Microscopic Features |
Ancillary Investigations |
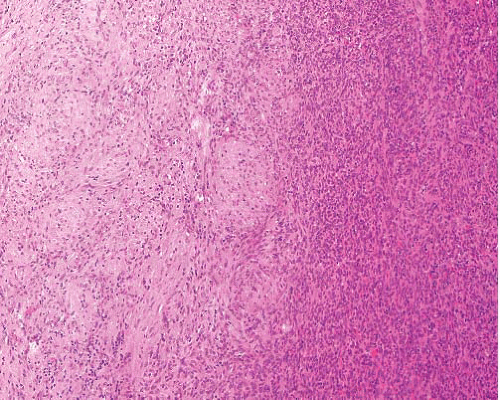
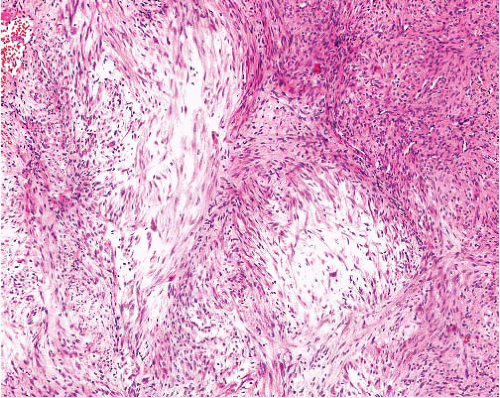
Myofibroma |
Mostly in children, occasionally in adults, in head and neck, extremities, bone
Dermal or subcutaneous
Occasionally visceral and multicentric
Mostly benign but deep multicentric forms can be fatal |
Circumscribed, occasionally central necrosis
Nodules of myofibroblasts, central smaller cells with pericytomatous pattern
Giant cells, ossification, intravascular spread |
SMA+, desmin−, h-caldesmon−, S100 protein−, CK− |
Nodular fasciitis |
Young adults, extremities, head and neck
Short history (weeks); rarely up to a year
Rapid growth then stops <5 cm |
Circumscribed or mildly infiltrative myofibroblastic tumor
Variably myxoid, cellular and collagenous areas
Normal mitoses acceptable but not atypia or necrosis |
SMA+, desmin±, h-caldesmon−, t(17;22)(p13;q22.1), USP6-MYH9 fusion |
Myopericytoma |
M > F, limbs, head and neck, trunk, in dermis, or subcutis
Single or multiple
Intravascular and malignant variants occur |
Circumscribed, encapsulated
Prominent pericytomatous pattern
Myoid or glomus cells around vessels or in nodules, or slender spindle cells in perivascular whorls
Malignant variants have infiltration, pleomorphism, mitoses, necrosis |
SMA+, caldesmon+, desmin−, CD34−, S100 protein−, CK−, t(7;12) (p21−22;q13−15), ACTB-GLI fusion |
Myoepithelial tumor/mixed tumor |
Adults, limbs and girdles, rarely head and neck or trunk
Dermis, subcutis, or deep soft tissue
Malignant variants occur |
Resembles salivary gland counterparts
Epithelial cords, nests, or sheets, plasmacytoid change, and rarely pure spindle cell pattern
Ductules in mixed tumor
Chondromyxoid stroma |
Variable expression of S100 protein, CK, EMA, GFAP, SMA, h-caldesmon, calponin
EWSR1 rearrangement by FISH± |
Mammary-type myofibroblastoma |
Breast M = F
Inguinal region M > F, buttock, abdominal wall
Painless, slow-growing, rarely >6 cm diameter
Benign |
Circumscribed tumor comprising irregular fascicles of spindle cells, collagenous bands, variable adipose tissue, mast cells
Resembles spindle cell lipoma
Atypical and epithelioid variants occur |
CD34+, desmin+, SMA±, h-caldesmon−, S100 protein−, CK− |
Intranodal myofibroblastoma |
Inguinal lymph node, rarely submandibular node
Very rarely recurs |
Rim of lymph node
Solid, hemorrhagic cut surface
Cellular fascicles of slender spindle cells with nuclear palisading
Extravasated erythrocytes, hemosiderin
Hyaline-walled vessels, amianthoid fibers |
SMA+, beta-catenin+, other markers negative |
Kaposi sarcoma, intranodal |
Children or young adults in lymph node draining skin lesion, or rarely primary generalized lymphadenopathy |
Curved fascicles of spindle cells, pleomorphism, and mitoses
Hemorrhage, hyaline globules |
HHV8+, CD34+, CD31+, ERG+, SMA−, S100 protein−, CK− |
Cellular schwannoma |
M > F
Deep soft tissue, retroperitoneum, pelvis, rare in lymph node
Firm mass up to 20 cm diameter
Can recur but does not metastasize |
Encapsulated (fragment of capsule often present in core biopsy), subcapsular lymphoid aggregates, sheets of elongated spindle cells with eosinophilic cytoplasm
Clusters of foamy macrophages, lymphocytes |
S100 protein+ diffusely, GFAP+, D2-40+, CK+ rarely, EMA+ (subcapsular) |
Fibromatosis—desmoid type |
Deep soft tissue, limbs, head and neck, body cavities |
Parallel-aligned myofibroblasts evenly dispersed in collagen, slitlike and thick-walled vessels, mast cells
No nuclear atypia or necrosis |
SMA+, beta-catenin+ in nuclei
CD34− |
Low-grade myofibrosarcoma |
Head and neck, extremities, retroperitoneum, bone, infiltrative mass, recurs |
Cellular fascicles infiltrate muscle
Myofibroblastic morphology, mostly uniform but focal nuclear atypia is diagnostic
Occasional necrosis |
SMA+, desmin±, h-caldesmon−, S100 protein−, CD34− |
Leiomyosarcoma |
Dermis, subcutis, or (mostly) deep soft tissue
Slowly growing
Some related to obvious blood vessel
Recurs |
Well-defined fascicles at right angles
Nontapered cells, eosinophilic cytoplasm, nuclear atypia, abnormal mitoses |
SMA+, desmin+, h-caldesmon+, CK in some (dot), CD34+ rarely |
|