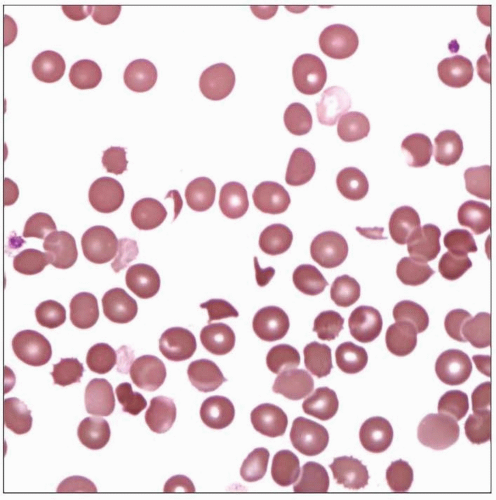
Microangiopathic Hemolytic Anemia
Qian-Yun Zhang, MD, PhD
Key Facts
Terminology
TTP is characterized by thrombocytopenia, microangiopathic hemolysis, and platelet microthrombi in multiple organ systems
HUS is characterized by progressive renal failure with associated microangiopathic hemolytic anemia and thrombocytopenia
DIC is characterized by uncontrolled thrombi formation in microvasculature due to disturbed hemostasis
Post-transplantation TMA follows allogeneic hematopoietic stem cell transplantation
Etiology/Pathogenesis
ADAMTS13 is severely deficient in TTP
Primary event in HUS is damage to endothelial cells; microthrombi formation
Tissue factor or bacterial toxin activation of coagulation cascade is primary event in DIC
Pathogenesis of post-transplantation TMA is most likely injury to endothelial cells
Clinical Issues
RBC fragmentation is common finding in all MAHA
Daily plasmapheresis (plasma exchange) is standard treatment for TTP
HUS usually shows positive stool culture
Supportive care is mainstay in HUS patients
DIC panel is positive in patients with DIC
Treating primary cause is paramount in DIC
Treating post-transplantation TMA is supportive
Diagnostic Checklist
MAHA is a diagnosis of clinicopathologic correlation
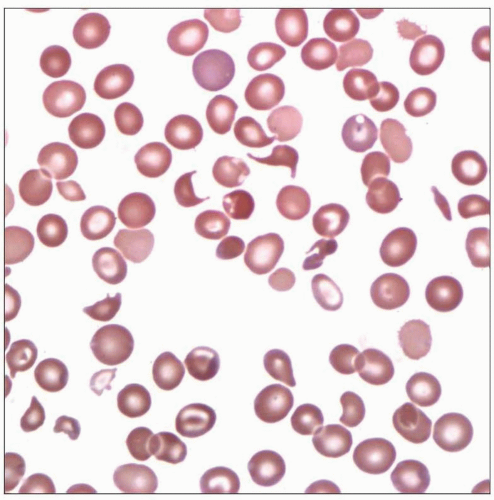 Blood smear of a young female with recent diagnosis of TTP reveals marked thrombocytopenia and frequent schistocytes. The patient underwent plasmapheresis and recovered fully. |
TERMINOLOGY
Abbreviations
Microangiopathic hemolytic anemia (MAHA)
Definitions
Fragmentation of red blood cells due to narrowing or obstruction of microvasculature
Major types and subtypes
Thrombotic thrombocytopenic purpura (TTP)
Congenital TTP
Acquired TTP
Hemolytic uremic syndrome (HUS)
Shiga-like (vero) toxin-associated HUS (Stx-HUS)
Non-Shiga-associated HUS (non-Stx-HUS) (a.k.a. atypical HUS or aHUS): Sporadic or familial
Disseminated intravascular coagulation (DIC)
Acute DIC
Chronic DIC
ETIOLOGY/PATHOGENESIS
Thrombotic Thrombocytopenic Purpura (TTP)
Etiology
Congenital TTP
Mutations of ADAMTS13 gene
Acquired TTP
Autoimmune disorders
Malignancy
Stem cell transplantation
Pregnancy (especially 3rd trimester)
Certain drugs (ticlopidine, mitomycin, clopidogrel, cyclosporine)
Infection, including HIV
Pathogenesis
Normal von Willebrand factor (vWF) homeostasis
vWF multimers are synthesized by endothelial cells and megakaryocytes
vWF are present in platelets, endothelial cells, and subendothelium
Protease ADAMTS13 cleaves ultra large vWF multimers into smaller vWF forms
VWF mediates platelet aggregation, activation, and thrombus formation at sites of vascular injury
Pathogenesis of TTP
Congenital TTP: Mutations lead to ADAMTS13 deficiency; episodes are triggered by infection, acute inflammation and pregnancy
Acquired TTP: Autoantibodies cause inhibition of ADAMTS13 activity
ADAMTS13 deficiency leads to accumulation of ultra large vWF multimers
Ultra large multimers have greater ability to react with platelets, causing disseminated platelet microthrombi
Pathogenesis unclear in some cases
Hemolytic Uremic Syndrome (HUS)
Etiology
Stx-HUS
Infections with Escherichia coli O157:H7 in 75% cases
Enterococcus or Shigella in some cases
Sporadic non-Stx-HUS cases
Infection with Streptococcus pneumonia in some cases
Familial non-Stx-HUS
Mutations in complement genes (factor H, membrane cofactor protein, factor I, factor B, C3)
Pathogenesis
Primary event in HUS is damage to endothelial cells and subsequent microthrombi formation
Stx-HUS
Exact pathogenesis unknown
Shiga toxin is thought to function as a molecular mimic of endothelial cell membrane bound molecule
Toxins bind to receptors on glomerular endothelial, mesangial, and tubular epithelial cells
Antibody to toxin cross-reacts with endothelial cells, resulting in endothelial cell damage and microvascular thrombosis
Non-Stx-HUS (aHUS)
Mutations lead to excessive complement activation on renal arterioles and interlobular arteries and interlobular arteries
Complement activation leads to endothelial damage, which leads to coagulation activation and thrombotic microangiopathy
Other triggers of non-Stx-HUS include nonenteric infections, viruses, drugs, malignancies, organ transplantation, and pregnancy
Disseminated Intravascular Coagulation (DIC)
Etiology
Infections: Gram positive or negative bacterial infections; rickettsial infection; viral infections
Tissue factor activation: Malignancies; tissue injury; extensive burn, brain injury
Other: Obstetric disorders; snakebite; vascular disorders; hemolysis
Pathogenesis
Tissue factor or bacterial toxin activates coagulation cascade resulting in disturbed hemostasis
Overproduction of thrombin leads to generation of fibrin monomers, which are crosslinked into insoluble fibrin polymers by FXIIIA
Fibrin polymers deposit in microvasculature (microthrombi); leads to tissue ischemia
Endothelial cell response to thrombi causes excessive fibrinolysis
Fibrin degradation products (FDP) from fibrinolysis interfere with platelet aggregation, potentiate bleeding risk
Depletion of platelets, fibrinogen, and other hemostatic proteins increase bleeding risk
Cytokines released by macrophages, monocytes, and endothelial cells may lead to shock
Other Types of MAHA
Post-transplantation thrombotic microangiopathy (post-transplantation TMA)
Poorly defined entity secondary to complications of allogeneic hematopoietic stem cell transplantation
Thrombosis limited to kidney
Pathogenesis is most likely injury to endothelial cells due to multiple possible factors
Diagnosis must exclude other causes of MAHA
Mechanical damage
Heart valve is classical example
Rarely seen in congenital heart malformation secondary to blood turbulence and mechanic damage
Miscellaneous causes of MAHA
Strenuous physical activity
Circulating mucinous material in disseminated carcinoma
Following chemotherapy
CLINICAL ISSUES
Presentation
TTP
TTP triad
Thrombocytopenia
Microangiopathic hemolytic anemia
Microthrombi of small vessels in multiorgan systems
Subtypes
Congenital TTP is rare, presents at birth/infancy
Acquired TTP is the predominant type
Clinical signs and symptoms
Insidious onset
Fluctuation of neurologic signs in most patients (headache, bizarre behavior, transient sensorimotor deficits, seizures, coma)
Fever
Petechiae on lower extremities
Renal impairment in minor subset of patients
Secondary DIC may arise from prolonged tissue ischemia
HUS
HUS triad
Thrombocytopenia
Microangiopathic hemolytic anemia
Progressive renal failure
Stx-HUS
Classic type; majority of cases
Disease of children younger than 2-3 years
Prodrome bloody diarrhea often present
Most common cause of acute renal failure in children
Non-Stx-HUS
Rare; accounts for 5-10% of HUS cases
Most commonly seen in early childhood, although may occur at any age
No prodromal bloody diarrhea and no Stx-producing E. coli infection
Streptococcus pneumoniae infection may lead to septicemia, meningitis, and pneumonia in some cases
Patient may present with hypertension
Some patients show central nervous system involvement at onset with convulsions and alterations of consciousness
Renal involvement consists of oliguria or anuria, high renal retention values, or degradation of creatinine clearance
DIC
Characterized by
Disturbed hemostasis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree