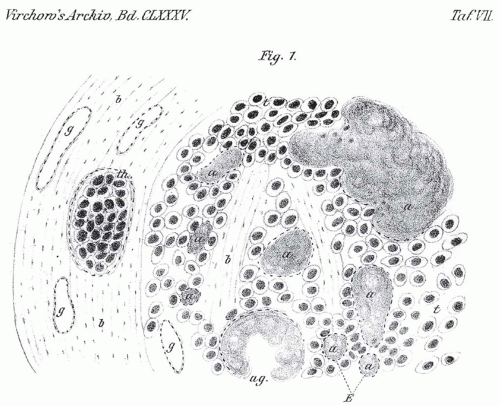
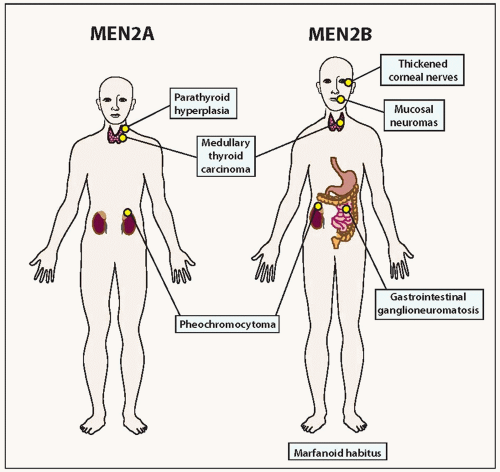
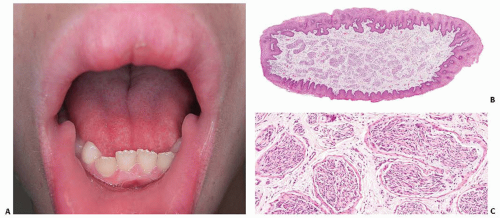
Germline RET Mutations
Familial medullary carcinomas are associated with gain-of-function mutations in the
RET gene. A 1985 study of a cell line transfected with human lymphoma DNA revealed a novel transforming gene.
42 This proved to be a fusion gene containing a portion of a gene that coded for a tyrosine kinase domain. This tyrosine kinase gene became known as
RET, its name a contraction acronym of
REarranged during
Transfection. Genetic linkage analysis localized the MEN2A locus to the centromeric region of chromosome 10 in 1987.
43 Subsequent studies revealed that
RET mapped to chromosome 10q11.2 and that germline mutations of
RET were the primary cause of MEN2A, MEN2B, and FMTC.
44,
45,
46 Most germline mutations are point mutations.
RET is the gene associated with both MEN2/FMTC disorders and Hirschsprung disease, but the sets of germline mutations are different. In contrast to activating mutations in hereditary medullary carcinoma, loss-offunction mutations are responsible for Hirschsprung disease.
36 Of note,
RET is also involved in the pathogenesis of papillary thyroid carcinoma, where a portion of the gene is fused and activated as a result of chromosomal rearrangement known as
RET/PTC (see
Chapter 11).
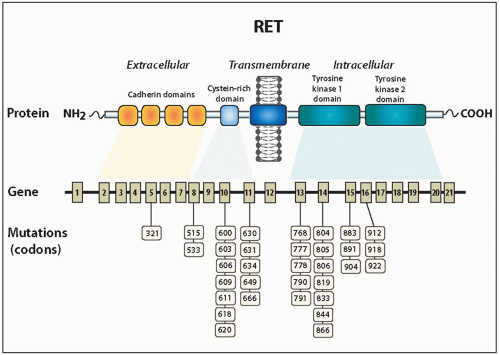
RET has 21 exons and approximately 55,000 base pairs.
47 It codes for a protein (RET) that is a member of the receptor tyrosine kinase superfamily. The receptor comprises an extracellular domain with calcium-dependent cell adhesion (cadherin) and cysteine-rich regions, a transmembrane domain, and an intracellular domain with regions of tyrosine kinase activity (
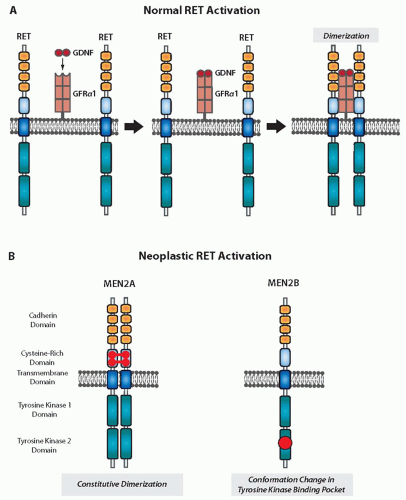
Fig. 14.4).
34
The RET receptor is activated when a complex ligand binds to the extracellular domain. Glial cell line-derived neurotrophic factor (GDNF) binds to GDNF family receptor α1, a glycosylphosphatidylinositol protein located on the cell surface, forming a complex that subsequently binds to RET (
Fig. 14.5).
36,
48,
49,
50 Binding of this complex results in receptor dimerization and autophosphorylation of tyrosine residues. This in turn initiates downstream signaling through the mitogen-activated protein kinase and other pathways.
34 Several other members of the GDNF ligand family have been shown to activate RET after binding to surface proteins. These include neurturin, artemin, and persephin.
36The RET receptor activates signaling pathways responsible for cell proliferation, survival, differentiation, motility, and chemotaxis.
34 RET is normally expressed by thyroid C cells and cells of the adrenal medulla, sympathetic ganglia, and kidneys. Populations of neural crest cells express RET during early embryogenesis and as they migrate to various regions of the body.
34 The normal development of the autonomic and enteric nervous systems and the excretory system is dependent on RET.
51Since the initial discovery of activating point mutations of RET as the primary cause of the hereditary forms of medullary carcinoma, a wide distribution of mutations have been identified in the extracellular cysteine-rich and the intracellular tyrosine kinase domains, as summarized in
Figure 14.4 and
Table 14.1.
33,
34,
35,
36,
41 Mutations of five cysteine-rich domain codons collectively account for about 95% of MEN2A and 85% of FMTC kindred.
35 Four are located on exon 10 (609, 611, 618, and 620) and one on exon 11 (634), and almost all mutations involve replacement of cysteine by another amino acid. Codon 634 is the most common site of mutations associated with MEN2A, being involved in 80% to 90% of cases. The most common mutation is substitution of arginine for cysteine (C634A), found in about 50% of MEN2A families.
34,
35,
41 The C634A mutation is also linked to parathyroid hyperplasia.
52 Most of the mutations associated with FMTC are found in extracellular cysteine-rich domain codons 618, 620, and 634 in a fairly even distribution.
35 FMTC also has been associated with mutations of the intracellular tyrosine kinase domain of
RET including codons 768, 790, 791, 804, 848, 883, 891, and 904.
35,
36,
41About 95% of cases of MEN2B are associated with a point mutation in the tyrosine kinase domain codon 918 of exon 16, resulting in the replacement of a methionine by threonine (M918T).
34,
41,
53,
54 A point mutation at codon 883 of exon 15 resulting in alanine replacement by phenylalanine accounts for almost all of the remaining small percentage of MEN2B cases.
34,
36,
55 A small number of MEN2B cases have been found to have double germline mutations involving codons 804+805, 804+806, and 804+904.
56,
57,
58,
59,
60 De novo mutations leading to MEN2B are more common compared with de novo mutations leading to MEN2A and FMTC.
Mutations in the cysteine-rich domain codons, which account for most cases of MEN2A and FMTC, activate the RET protein by constitutive dimerization (
Fig. 14.5). The mutated forms of RET have unpaired cysteine residues that can form disulfide bonds with other RET monomers, and thus, dimerization occurs without the necessity of a ligand.
36,
61,
62 Mutation of the 918 codon, which is associated with MEN2B, affects the catalytic core region of the intracellular tyrosine kinase domain and causes constitutive
activation of the monomeric form of RET.
36,
54 A conformational change in the binding pocket of the tyrosine kinase domain leads to an altered substrate specificity. A small portion of FMTC cases have mutations involving the intracellular tyrosine kinase domain. The activation mechanisms associated with these mutations are not well defined at this time.
Other Genetic Abnormalities
Other genetic mutations and/or epigenetic events are likely to play a role in the development of sporadic and hereditary medullary carcinoma. The disease phenotype of hereditary tumors generally correlates with
RET mutations of specific codons, but individuals with the same germline mutation still show some
variation in regard to clinical manifestations of disease. Several potential oncoproteins and tumor suppressors are present in the signaling pathways utilized by the RET receptor, and mutations in genes encoding these proteins may play significant roles in the development of medullary carcinoma. Major signaling proteins activated downstream of RET are RAS and phosphatidylinositol 3-kinase. As discussed above,
RAS mutations have been found in a significant number of sporadic medullary carcinomas. Loss
of function of negative regulators of RET signaling such as the tyrosine phosphatases LAR, PTPRJ, or SHP-1 is another potential mechanism of tumorigenesis. Murine studies suggest that mutations of the tumor suppressor genes
RB1 and
TP53 may play a role in medullary carcinoma.
Loss of Heterozygosity
Analyses of both hereditary and sporadic cases of medullary carcinoma have shown loss of heterozygosity at one or more chromosomes other than 10q including 1p, 3p, 3q, 11p, 11q, 13q, 17p, 18p, 18q, 19p, 19q, and 22q.
70,
71,
72 About 25% of hereditary and 75% of sporadic cases show imbalances when analyzed by comparative genomic hybridization (CGH).
70 Medullary thyroid carcinomas with the somatic
RET M918T mutation tend to have the highest number of chromosomal imbalances detected by CGH.
71 These chromosomal imbalances are due to both gains (amplification) and losses (deletions). The affected regions are known to be the location of tumor suppressor genes as well as the genes that code for proteins of the GDNF family of receptors and their ligands.
70 The developing picture of pathogenesis suggests that
RET mutations cause C-cell hyperplasia, at least in familial cases, creating a favorable environment for the development of medullary carcinoma and that additional somatic mutations lead to expansion of clones with more aggressive biologic properties.
70