Some Basic Concepts, Terms, and Epidemiology
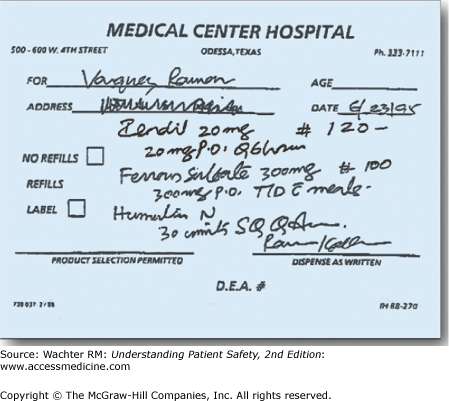
In June 1995, a middle-aged man named Ramon Vasquez went to see his physician in Odessa, Texas, to investigate his chest pain. His physician, suspecting angina, prescribed a medication. The actual prescription is reproduced in Figure 4-1.
Plendil, a calcium channel blocker sometimes used to treat angina?
Isordil, a long-acting nitrate also used to treat angina?
Zestril, an angiotensin-converting enzyme inhibitor used to treat high blood pressure and heart failure?
So what did you think? I once asked an audience of hospitalists to interpret this prescription. Half said it was Plendil, one-third Isordil, and the rest Zestril.
The physician actually intended to prescribe 120 tablets of Isordil, at its typical dose of 20 mg by mouth (po) every (Q) 6 hours. Ramon Vasquez’s pharmacist read the prescription as Plendil, and instructed the patient to take a 20-mg pill every 6 hours. Unfortunately, the usual starting dose of Plendil is 10 mg/day, making this an eightfold overdose. A day later, Mr. Vasquez was critically ill from low blood pressure and heart failure. He died within the week.
The modern pharmaceutical armamentarium represents one of healthcare’s great advances. There are now highly effective agents to treat most common maladies: high blood pressure and cholesterol, diabetes, heart disease, cancer, stroke, acquired immunodeficiency syndrome (AIDS), and more. Taken correctly, the benefits of these medications far outweigh their side effects, though the latter remains a concern even when medications are prescribed and taken correctly.
But the growth in medications (there are now more than 10,000 prescription drugs and biologicals—and 300,000 over-the-counter products—available in the United States1) has led to a huge increase in the complexity of the medication prescribing and administration process. It has been estimated that at least 5% of hospital patients experience an adverse drug event (ADE; harm experienced by a patient as a result of a medication, from either a side effect or the consequence of an error) at some point during hospitalization. Another 5–10% experience a potential ADE, meaning that they nearly took the wrong medicine or the wrong dose but didn’t, often thanks to a last minute catch or dumb luck.2 When patients are on high-risk medications such as warfarin, insulin, and heparin, errors are particularly common: in a national sample of hospitalized Medicare patients, one in seven receiving heparin experienced an ADE.3 The cost of preventable medication errors in U.S. hospitals has been estimated at $16.4 billion annually.4
Things are no safer outside the hospital. Nearly 1 in 20 hospital admissions can be traced to problems with medications, many of them preventable.5 When a large group of outpatients on a variety of medications was followed for 3 months, about one in four suffered an ADE, many of them serious.6 The cost of ambulatory preventable medication errors is nearly $5 billion annually,7 bumping the total cost of medication errors to over $20 billion each year.4
Although many discussions about medication errors focus on the famous illegibility of physicians’ handwriting, two studies have shown that doctors’ handwriting is no worse than that of many other professionals,7,8 and—notwithstanding the tragic case of Ramon Vasquez—bad handwriting is not a common cause of medication errors. In fact, many steps along the medication prescribing and administration pathway are vulnerable to mistakes. To illustrate these steps, let’s follow the life of an inpatient prescription (in a hospital without computerized order entry or bar code medication administration):
- A physician handwrites a prescription in the “Doctors’ Orders” section of the chart.
- The clerk removes a carbon copy of the order and faxes it to the pharmacy, while a nurse transcribes the order into the Medication Administration Record (MAR).
- A pharmacist receives the faxed copy, reads it, and retypes the medication, dose, and frequency into the pharmacy’s computer system, which generates labels and a bill and helps track inventory.
- The pharmacist manually transfers the medication (if a pill) from a large bottle into “unit doses”—either cups or a shrink-wrapped container. Intravenous medication may require specialized mixing.
- The medication is delivered to the patient’s floor; the label includes the name of the medication and the patient’s name. The medicine may be delivered on a cart wheeled to the floor, or through a manual transport or pneumatic tube system.
- The nurse goes to the MAR, sees that the patient is due for a medication, searches the medication cart for it, and walks to the patient’s room with the medication (along with different medications for her other patients).
- The nurse enters the patient’s room, confirms the patient’s identity, checks the medication, and administers it.
Believe it or not, I have simplified this process to avoid taking up too much space. Several hospitals have found that there are actually about 50–100 steps between a doctor’s decision to order a medicine and the delivery of the medicine to the patient. The outpatient process is simpler, but only by a bit. There, the doctor usually gives the patient a paper prescription to carry to a pharmacy. Not only can there be errors in the prescription itself (wrong medicine, wrong dose, illegible prescription, failure to consider allergies or drug–drug, drug–disease, or drug–diet interactions), the administration of the medication (generally a mistake in the pharmacy), or a failure to monitor properly (forgetting to check electrolytes and creatinine in a patient started on a diuretic and angiotensin-converting enzyme inhibitor), but also a new source of errors is introduced: patients themselves (e.g., failure to follow instructions properly or storing the medications in incorrect bottles; Chapter 21).9 With all these steps, a statistical law takes its toll: in a 50-step process in which each step is done correctly 99% of the time, the chance that at least one error will occur is a staggering 39%!
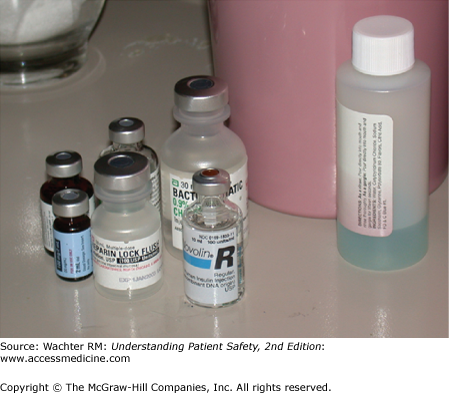
Sarah Geller was a 68-year-old woman who had undergone a cardiac bypass operation. After a stormy postoperative course she seemed to be on the road to recovery. However, on the morning of her planned transfer out of the intensive care unit (ICU), she suffered a grand mal seizure. This shocked her caregivers: she had no seizure history and was not on any epileptogenic medications. They drew some blood tests, and emergently wheeled her to the computed tomography (CT) scanner to rule out the possibility of a stroke or intracerebral hemorrhage. While she was in transit, the lab paged the doctors to report that Geller’s serum glucose was undetectable. Despite multiple infusions of glucose, she never recovered from her coma. In the subsequent investigation, it was determined that her bedside tray in the ICU contained vials of both heparin (used to “flush” her intravenous lines to keep them open) and insulin. The vials were of similar size and shape (Figure 4-2). The nurse, intending to flush Ms. Geller’s line with heparin, had inadvertently administered a fatal dose of insulin.10
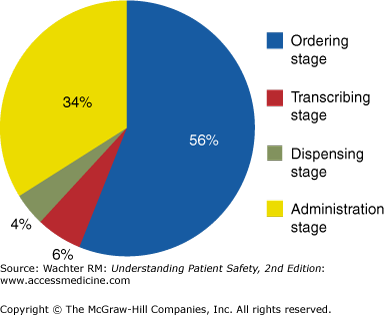
As this case demonstrates, solutions to the problem of medication errors will need to tackle both the prescribing and the administration phase1,2 (Figure 4-3). Many of the solutions will be technological: the role of computerized provider order entry (CPOE), computerized decision support, and bar coding and/or radio-frequency identification (RFID) systems will be discussed in Chapter 13. The remainder of this chapter will focus on solutions more specific to the medication prescribing and administration process: standardization, vigilance and the “Five Rights,” double checks, preventing interruptions and distractions, unit dosing, removal of risky medications from certain settings, the role of clinical pharmacists, and meeting the challenges of look-alike, sound-alike medications. A final section will review the main strategy to avoid medication errors at the time of transitions: medication reconciliation.
Figure 4-3
Medication errors, by stage of the medication process. (Reproduced with permission from Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995;274:29–34. Copyright © 1995 American Medical Association. All rights reserved.)
Strategies to Decrease Medication Errors
Betsy Lehman, a popular Boston Globe health columnist, was hospitalized for recurrent breast cancer at Dana-Farber Cancer Institute in 1994. Her experimental protocol called for her to receive an unusually high dose of cyclophosphamide (a chemotherapy agent), followed by a bone marrow transplant. The ordering physicians wrote a prescription: “cyclophosphamide 4 g/sq m over four days,” intending that she receive a total of 4 g/m2 of body surface area spread out over 4 days. Instead, the nurses administered the total dose (4 g/m2) on each of the 4 days, a fourfold overdose. She died within a month.
The Lehman case, one of the catalysts for the modern patient safety movement (Table 1-1), can be seen as an argument for computerization and decision support. It would be easy to envision a computer system preprogrammed with the correct dose of chemotherapy that either presented that dose as a default option to the physicians or automatically alarmed when someone prescribed an out-of-range dose.11 But the case also screams out for standardization: general agreements on inviolable ways of communicating certain orders that would be understandable to everyone.12 For example, a hospital could mandate that all medications given over multiple days must have the daily dose written each day. Or that high-risk medications could only be ordered one day at a time.
| Do Not Use | Potential Problem | Use Instead |
|---|---|---|
| U (unit) | Mistaken for “0” (zero), the number “4” (four), or “cc” | Write “unit” |
| IU (International Unit) | Mistaken for IV (intravenous) or the number 10 (ten) | Write “International Unit” |
| Q.D., QD, q.d., qd (daily) | Mistaken for each other | Write “daily” |
| Q.O.D., QOD, q.o.d, qod (every other day) | Period after the Q mistaken for “I” and the “O” mistaken for “I” | Write “every other day” |
| Trailing zero (X.0 mg) | Decimal point is missed | Write X mg |
| Lack of leading zero (.X mg) | Write 0.X mg | |
| MS | Can mean morphine sulfate or magnesium sulfate | Write “morphine sulfate” |
| MSO4 and MgSO4 | Confused for one another | Write “magnesium sulfate” |
One source of ambiguity has been the long-standing use of abbreviations for certain medications. In 2004, the Joint Commission prohibited hospitals from using a group of “high-risk abbreviations” (Table 4-1), insisting that the full name of these medications and instructions (“morphine sulfate,” not “MS04”; “Insulin 10 Units,” not “Insulin 10 U”) be spelled out. One of the advantages of CPOE will be to further standardize nomenclature and markedly limit the use of abbreviations. However, as will be further discussed in Chapter 13, CPOE has the capacity to create new classes of medication errors if it is not well designed and implemented.13,14 For example, unless sophisticated decision support is built into the system, a provider intending to prescribe “penicillin” can easily select the anti-inflammatory agent “penicillamine” from an alphabetical computerized pick list.15
While many advances in medication safety have come from system changes such as automation and double checks, individual caregivers are responsible for doing what they can to ensure the safety of their patients. One long-standing nursing principle is known as the “Five Rights” (Table 4-2), highlighting the five checks that a nurse is expected to make before administering a medication to a patient. Recently, some have argued for additional checks, including right documentation, right action (i.e., the medication is being given for the correct indication), right form (i.e., oral vs. intravenous, can the pill be safely crushed?), and right response (i.e., the nurse should monitor the patient’s response to the medication).16 Some add still another: the right of a patient to refuse a medication. Although these additional rights have not yet become standard practice, they further illustrate the tremendous challenges of correct medication administration and the limitations of human vigilance as a safety bulwark.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree