Objectives
- Describe the physiologic functions of the principal components of the male reproductive system.
- Describe the endocrine regulation of testicular function by gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone, testosterone, and inhibin.
- Identify the cell of origin for testosterone, its biosynthesis, mechanism of transport within the blood, metabolism, and clearance. List other physiologically produced androgens.
- List the target organs or cell types, the cellular mechanisms of action, and the physiologic effects of testosterone.
- Describe spermatogenesis and the role of different cell types in this process.
- Understand the neural, vascular, and endocrine factors involved in the erection and ejaculation response.
- Compare and contrast the actions of testosterone, dihydrotestosterone, estradiol, and müllerian inhibitory factor in the process of sexual differentiation.
- Identify the causes and consequences of androgen oversecretion and undersecretion in prepubertal and postpubescent adult males.
Male Reproductive System: Introduction
In utero sexual differentiation, maturation, spermatogenesis, and ultimately reproduction are all functions of the male reproductive system that are under endocrine regulation. The 2 principal functions of the testicles, the adult male sex organs, are the production of sperm and the synthesis of testosterone. These processes ensure fertility and maintain male sexual characteristics, or virility. Testicular function is under central nervous system control in a classic neuroendocrine feedback loop with the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH) as the key hormonal signals. These gonadotropins, as discussed in Chapter 2, are under the influence of hypothalamic gonadotropin-releasing hormone (GnRH) stimulation. Additional paracrine, neural, and endocrine factors contribute to the complex regulation of the hypothalamic-pituitary-gonadal axis. This chapter discusses the basic principles of endocrine regulation of the male reproductive system.
Functional Anatomy
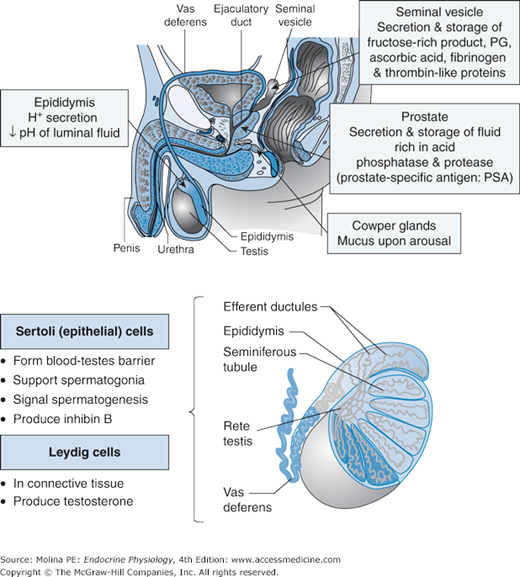
![]() The male reproductive organs include the testes (the central male sex organs), the vas deferens, the ejaculatory ducts, the penis, and the accessory glands, which include the prostate and bulbourethral glands (Figure 8–1). The testes consist of numerous lobules made of convoluted tubes (tubuli seminiferi) supported by loose connective tissue. The seminiferous tubules represent approximately 80%–85% of the testicular mass or volume. The tubules consist of a basement layer lined with epithelial (Sertoli) cells forming the walls of the seminiferous tubules. These tubules are lined with primitive germ cells (spermatogonia). The Leydig cells are embedded in the connective tissue are the endocrine cells responsible for the production of the most important circulating androgen, testosterone.
The male reproductive organs include the testes (the central male sex organs), the vas deferens, the ejaculatory ducts, the penis, and the accessory glands, which include the prostate and bulbourethral glands (Figure 8–1). The testes consist of numerous lobules made of convoluted tubes (tubuli seminiferi) supported by loose connective tissue. The seminiferous tubules represent approximately 80%–85% of the testicular mass or volume. The tubules consist of a basement layer lined with epithelial (Sertoli) cells forming the walls of the seminiferous tubules. These tubules are lined with primitive germ cells (spermatogonia). The Leydig cells are embedded in the connective tissue are the endocrine cells responsible for the production of the most important circulating androgen, testosterone.
Figure 8–1.
Functional anatomy of the male reproductive system. The male reproductive organs include the testes, the vas deferens, the ejaculatory ducts, the penis, and the accessory glands, which include the prostate and bulbourethral glands. The testes consist of numerous lobules made of tubuli seminiferi supported by loose connective tissue. The tubuli seminiferi are united to form larger ducts called the tubuli recti. These larger tubules form a close anastomosing network of tubes called the rete testis, terminating in the ductuli efferentes. The tubular network carries the seminal fluid from the testis to the epididymis, from where spermatozoa enter the vas deferens and then enter the urethra through the ejaculatory ducts. The penis is composed of 2 functional compartments: the paired corpora cavernosa and the corpus spongiosum. The corpora cavernosa form the greater part of the substance of the penis and consist of bundles of smooth muscle fibers intertwined to form trabeculae, and containing numerous arteries and nerves. PG, prostaglandins. (Reproduced with permission from Widmaier EP, Raff H, Strang KT, eds. Vander’s Human Physiology: The Mechanisms of Body Function. 11th ed. New York, NY: McGraw-Hill; 2007: figures 17–5 and 17–6.)
Sertoli cells form tight junctions creating a “blood-testis” barrier that functionally divide the seminiferous tubules into 2 compartments or environments for the development of spermatozoa. The basal compartment below the tight junctions has contact with the circulation and is the space in which spermatogonia develop to primary spermatocytes. The tight junctions open at specific times and allow progression of spermatocytes to the adluminal compartment, where meiosis is completed. The principal functions of Sertoli cells are as follows:
- Provide support for germ cells, forming an environment in which they develop and mature.
- Provide the signals that initiate spermatogenesis and sustain spermatid development.
- Regulate pituitary gland function and, in turn, control of spermatogenesis.
Together, the Sertoli and Leydig cells are the 2 principal cell types responsible for testicular function.
The tubuli seminiferi are united to form larger ducts called tubuli recti. These larger tubules form a close anastomosing network of tubes called the rete testis, terminating in the ductuli efferentes (see Figure 8–1). This tubular network carries the seminal fluid containing sperm from the testis to the epididymis; from here, spermatozoa enter the vas deferens and then the ejaculatory ducts. The ejaculatory ducts move semen (sperm containing fluid) into the urethra. The tubular network or excretory system and the accessory organs contribute to the final composition of sperm-containing semen through absorptive and secretory processes (summarized in Table 8–1). Sperm constitutes approximately 10% of the volume of the ejaculated semen, which is composed of testicular and epididymal fluid together with the secretory products of male accessory glands. Most of the volume of the ejaculate is formed by the seminal vesicles with the remainder consisting of epididymal fluids and secretions from the prostrate gland and bulbourethral glands.
| Organ | Function |
|---|---|
| Excretory system | |
| Efferent ductules, vas deferens, ejaculatory duct, urethra |
|
| Epididymis |
|
| Accessory glands | |
| Seminal vesicle | Secretion and storage of fructose-rich product (preferred energy substrate for sperm), prostaglandins, ascorbic acid, fibrinogen-like and thrombin-like proteins |
| Prostate | Secretion and storage of fluid rich in acid phosphatase and protease (prostate-specific antigen) |
| Cowper glands | Secretion of mucus into the urethra upon arousal |
The penis is composed of 2 functional compartments: the paired corpora cavernosa and the corpus spongiosum. The corpora cavernosa form the greater part of the substance of the penis and consist of bundles of smooth muscle fibers intertwined in a collagenous extracellular matrix. Interspersed within this parenchyma is a complex network of endothelial cell–lined sinuses, or lacunae, arteries, and nerve terminals. The penis is innervated by somatic and autonomic (both sympathetic and parasympathetic) nerve fibers. The somatic innervation supplies the penis with sensory fibers and supplies the perineal skeletal muscles with motor fibers. Autonomic nerves mediate vascular dilation leading to penile erection, stimulate prostatic secretions, and control smooth muscle contraction of the vas deferens during ejaculation.
The arterial blood supply to the male reproductive organs is predominantly derived from the superficial and deep external pudendal branches of the femoral artery, the superficial perineal branch of the internal pudendal artery, and the cremasteric branch from the inferior epigastric artery. Venous drainage follows the course of the corresponding arteries. Lymphatics drain into the inguinal lymph glands.
Gonadotropin Regulation of Gonadal Function
![]() The primary functions of the testes are to produce spermatozoa and to produce the hormones involved in the regulation of reproductive function and virilization. These functions are regulated by the pituitary gonadotropins FSH and LH. LH and FSH circulate unbound in the plasma and have a half-life of 30 minutes (LH) and 1–3 hours (FSH). LH has higher amplitude fluctuations in plasma than FSH; FSH levels are more stable and show less variability.
The primary functions of the testes are to produce spermatozoa and to produce the hormones involved in the regulation of reproductive function and virilization. These functions are regulated by the pituitary gonadotropins FSH and LH. LH and FSH circulate unbound in the plasma and have a half-life of 30 minutes (LH) and 1–3 hours (FSH). LH has higher amplitude fluctuations in plasma than FSH; FSH levels are more stable and show less variability.
The gonadotropins produce their physiologic responses by binding to cell membrane Gαs protein–coupled receptors located in the Leydig and Sertoli cells, leading to activation of adenylate cyclase and increased formation of cyclic 3′,5′-adenosine monophosphate (cAMP) (Figure 8–2; see Figure 1–5). The increase in intracellular cAMP results in the activation of protein kinase A and subsequent kinase-mediated protein phosphorylation, which mediates the cellular effects of the gonadotropins. This process is similar to that used for adrenocorticotropic hormone–mediated stimulation of adrenal steroid hormone production (discussed below).
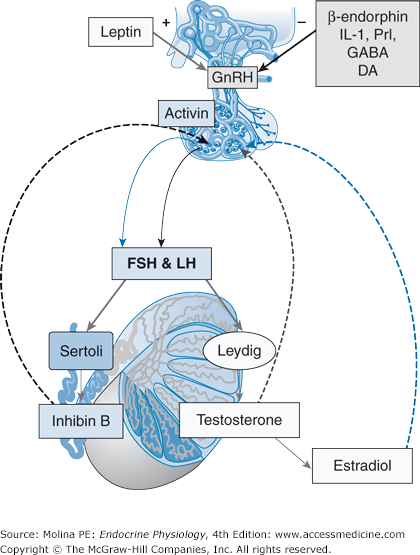
Figure 8–2.
Negative feedback regulation of gonadotropin synthesis and release. Gonadotropin release from the anterior pituitary gland is controlled by the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator. Factors that stimulate GnRH release include norepinephrine (NE), neuropeptide Y (NPY), and leptin. Factors that inhibit GnRH release include β-endorphin, interleukin 1 (IL-1), γ-aminobutyric acid (GABA), and dopamine (DA) neurons. The activity of the pulse generator and the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are regulated by the gonadal hormones testosterone and inhibin B and by locally produced factors such as activin. Activin interacts with inhibin B, thus increasing FSH β-subunit synthesis. The negative feedback regulation exerted by testosterone is mediated by local conversion to 17β-estradiol.
LH is the principal regulator of testosterone production by the Leydig cells. FSH plays an important role in the development of the immature testis, particularly by controlling Sertoli cell proliferation and seminiferous tube growth. Because the tubules account for approximately 80% of the volume of the testis, FSH is of major importance in determining testicular size, normally 4.1–5.2 cm in length and 2.5–3.3 cm in width in the adult male. FSH is important in the initiation of spermatogenesis during puberty. It is necessary for the production of androgen-binding protein by Sertoli cells and for the development of the blood-testis barrier.
![]() The overall regulation of FSH and LH secretion from the anterior pituitary was discussed in Chapter 3. The synthesis and release of the gonadotropins are regulated by neuroendocrine signals from the central nervous system, particularly the hypothalamus through the pulsatile release of GnRH, as well as by circulating hormones or their metabolites, as illustrated in Figure 8–2.
The overall regulation of FSH and LH secretion from the anterior pituitary was discussed in Chapter 3. The synthesis and release of the gonadotropins are regulated by neuroendocrine signals from the central nervous system, particularly the hypothalamus through the pulsatile release of GnRH, as well as by circulating hormones or their metabolites, as illustrated in Figure 8–2.
Pulsatile release of GnRH is determined by a pulse generator. The neuronal structures and chemical interactions that result in pulsatile GnRH release have not been fully elucidated. However, several central and peripheral signals modulate the activity of GnRH-releasing neurons. Some of these signals are stimulatory to GnRH release, such as norepinephrine and neuropeptide Y; others are inhibitory, such as β-endorphin and interleukin 1; others are both stimulatory and inhibitory, such as the estrogen 17β-estradiol. GnRH binds to a G protein–coupled receptor (Gq and G11) in the anterior pituitary gonadotrophs, activating phospholipase C and leading to the stimulation of inositol trisphosphate, diacylglycerol and protein kinase C. Activation of inositol trisphosphate leads to an increase in intracellular Ca2+ concentrations. GnRH also indirectly stimulates cAMP, contributing to control of LH and FSH release. The ratio between LH and FSH production is determined by the frequency of GnRH pulses. Synthesis of FSH β-subunit is highest in response to low-frequency pulses of GnRH and is suppressed at higher-frequency pulses of GnRH. Higher frequency and amplitude of GnRH stimulation increase LH β-subunit synthesis.
LH stimulates testosterone production by the Leydig cell. Testosterone released into the circulation inhibits LH release in a negative feedback loop. At the level of the hypothalamus, testosterone inhibits GnRH release, and at the anterior pituitary, it decreases the gonadotropin-specific β-subunit synthesized (see Figure 8–2). Testosterone reduces LH levels and the LH pulse amplitude. It is important to mention that most of the inhibitory effect of testosterone on LH release is mediated by 17β-estradiol, a locally produced metabolite of testosterone aromatization (Figure 8–3). The negative feedback inhibition of FSH release occurs at the level of the pituitary and is mediated mainly by inhibin B, a Sertoli cell–derived peptide (discussed later).
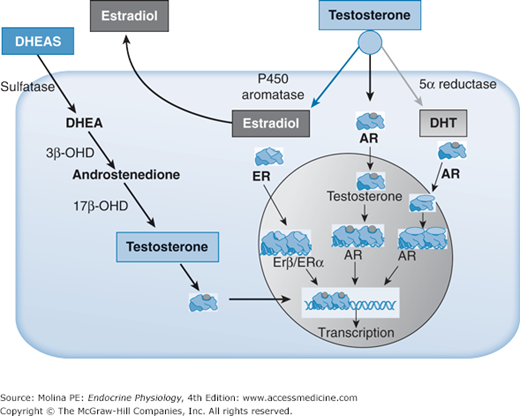
Figure 8–3.
Receptor-mediated effects of testosterone at target tissues. Testosterone (a steroid hormone) enters the cell by passive diffusion and binds to the androgen (AR) receptor. Testosterone can be converted to dihydrotestosterone (DHT) by 5α-reductase and bind to the AR or it can be converted to 17β-estradiol by aromatase and either be released to act on a neighboring cell’s estrogen receptors (ER) (paracrine mechanism), it can enter the circulation (endocrine effects), or it can bind to either the ER α or β. Intracellular testosterone can arise from androstenedione (Δ4A), dehydroepiandrosterone (DHEA), or dehydroepiandrosterone sulfate (DHEAS). The desulfated DHEA is converted to androstenedione by 3β-hydroxysteroid dehydrogenase (3β-OHD), and androstenedione transformed into testosterone by 17β-hydroxysteroid dehydrogenase (17β-OHD). Testosterone, DHT, and estradiol all bind to cytosolic steroid receptors. The cytosolic AR and ER is complexed to regulatory proteins (heat-shock proteins). Hormone binding results in the dissociation of the heat-shock protein complex, dimerization of the receptor, nuclear translocation, and DNA binding to regulatory elements. The final result is the activation of gene transcription.
In addition to the traditional feedback inhibition of gonadotropin hormone release described above (see Figure 8–2), locally produced factors (inhibin and activin) are also involved in regulation of gonadotropin release. Inhibins are peptide hormones that belong to the transforming growth factor beta (TGF-β) superfamily of growth factors. Inhibin B is produced by Sertoli cells in response to FSH stimulation (discussed later), and it produces feedback inhibition of FSH β-subunit production (and thus, FSH release). An additional factor involved in the regulation of FSH release is activin. Activin is expressed in several tissues including the pituitary. Its role is to antagonize inhibin B action, resulting in stimulation of FSH release. Thus, in addition to the negative feedback inhibition produced by gonadal androgens, the interaction between inhibin and activin contributes to the regulation of gonadotropin release.
Gonadal Function
The 2 principal physiologic functions of the testes—the production of hormones involved in sexual differentiation, maturation, and virilization and spermatogenesis—are closely related to each other. This chapter first discusses hormone production in the testes and then describes the process of spermatogenesis.
![]() The 3 principal hormones produced by the testis are testosterone, estradiol, and inhibin.
The 3 principal hormones produced by the testis are testosterone, estradiol, and inhibin.
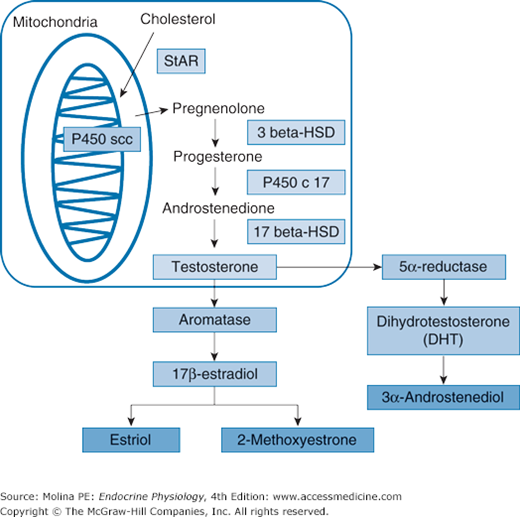
Testosterone, produced by the Leydig cells, is the principal and most important testicular and circulating androgen. LH stimulates testosterone biosynthesis by increasing mobilization and transport of cholesterol into the steroidogenic pathway, an action that takes place within minutes; as well as by stimulating gene expression and activity of the steroidogenic enzymes (steroidogenic acute regulatory protein and P450scc), a slower process that requires several hours (Figure 8–4). As discussed in Chapter 6, steroidogenic acute regulatory protein (also found in the adrenal cortex cells) has a key role in the transfer of cholesterol from the outer to the inner mitochondrial membrane, the first step in steroid hormone biosynthesis, for the conversion of cholesterol to pregnenolone. Pregnenolone diffuses out of the mitochondria to the smooth endoplasmic reticulum, where it is further metabolized by the action of 3β-hydroxysteroid dehydrogenase to progesterone. Progesterone in turn is converted by a 2-step process to androstenedione by the action of 17α-hydroxylase. The conversion of androstenedione to testosterone is catalyzed by 17β-hydroxysteroid dehydrogenase. Note that up until this last enzymatic reaction, the enzymatic steps involved in testosterone synthesis are similar to those involved in androstenedione synthesis by the adrenal glands (see Figure 6–3). It is the activity of 17β-hydroxysteroid dehydrogenase and the enzymatic conversion of androstenedione to testosterone that are specific to the gonads.