Major Hepatic Resection
Neal Wilkinson
Hepatic resections are performed for benign and malignant conditions. Hepatic adenoma, hemangioma, and focal nodular hyperplasia are all benign mass lesions that the surgeon may be asked to manage. With improved noninvasive imaging, to include computed tomography, ultrasound, and magnetic resonance imagery, resection for diagnostic purposes is seldom required. The treatment of cystic lesions: Simple, complex, neoplastic, and infectious needs to be individualized and may range from observation, simple marsupialization, to resection. Traumatic liver injury can lead to acute and delayed bleeding, infection, and bile leaks. Urgent surgical management is limited to obtaining control of ongoing bleeding. Damage control surgery is now recommended over anatomic resection at time of injury to avoid death from hypotension, hypothermia, and coagulopathy.
Major hepatic resections are most commonly performed for malignant tumors including primary hepatocellular carcinoma, primary biliary adenocarcinoma often referred to as intrahepatic cholangiocarcinoma, and a wide variety of metastatic lesions.
SCORE™, the Surgical Council on Resident Education, classified open segmentectomy/lobectomy of the liver as “COMPLEX” procedures.
STEPS IN PROCEDURE
Common Steps for All Resections
Right subcostal or bilateral subcostal (optional sternal split)
Divide falciform ligament cephalad to hepatic veins
Gently rotate left liver medially and down and incise triangular ligament
Roll liver medially to expose and divide right triangular ligament to hepatic veins
Elevate left lateral liver to expose caudate lobe and divide transparent part of lesser omentum
Completely surround porta hepatis through foramen of Winslow, place umbilical tape in case vascular control is later required (Pringle maneuver)
Wedge Resections
Confirm that lesion is amenable to wedge (as apposed to formal segmental) resection
Outline wide margins (1 to 2 cm) on lesion
Place through-and-through 2-0 chromic sutures just beyond planned margin and tie these gently
Sharply excise wedge of tissue
Obtain hemostasis
Left Lateral Bisegmentectomy (Segments II and III)
Elevate these segments up off of the caudate lobe to expose narrowest segment (transition point)
Divide small vascular pedicles to immediate left of falciform
Divide liver parenchyma from anterior to posterior along narrow transition point; secure left hepatic vein
Complete the transection and remove the specimen
Obtain hemostasis
Right Hepatectomy (Segments V to VIII)
Remove gallbladder and trace cystic duct to common hepatic duct (CHD)
Follow anterior surface of CHD to right hepatic duct, secure and divide
Identify and control right hepatic artery
Retract stumps of right hepatic duct and hepatic artery to the left to expose right portal vein
Carefully dissect, control, and divide the right portal vein
Reflect the entire liver medially to expose the vena cava; sequentially control and divide retrohepatic branches to cava
Divide parenchyma along line of demarcation, controlling any small vessels or bile ducts
Obtain hemostasis and bile stasis
Left Hepatectomy (Segments II through IV)
Remove gallbladder and trace cystic duct to CHD, then to left hepatic duct
Divide left hepatic duct
Expose, control, and divide left hepatic artery and left portal vein
Rotate liver downward to expose left and middle hepatic veins; control and divide these
Divide liver along line of demarcation, controlling any small vessels or bile ducts
Obtain hemostasis and bile stasis
Place omentum into operative field and close abdomen without drains
HALLMARK ANATOMIC COMPLICATIONS
Injury to artery, duct, or portal vein supplying remnant to be left behind
Massive bleeding from failure of vascular control
Hepatic insufficiency due to resection
Bile leak
LIST OF STRUCTURES
Left and right triangular ligaments
Diaphragm
Falciform ligament
Left, right, and middle hepatic veins
Portal Vein
Left and right portal veins
Common bile duct
Left and right hepatic ducts
Common and proper hepatic arteries
Left and right hepatic arteries
Glisson’s capsule
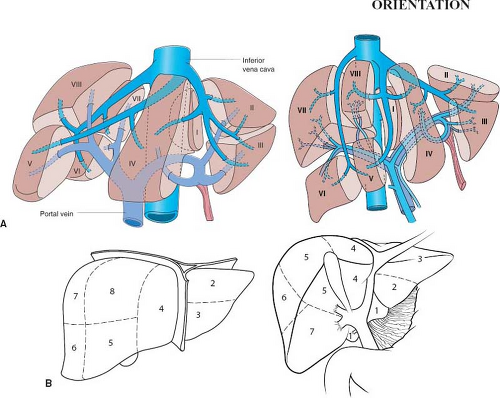
Hepatic segmental anatomy
For malignant lesions, a safe and planned surgical intervention is required to ensure appropriate margins, adequate hepatic reserve, and low surgical morbidity and mortality. Key elements to a successful surgical plan must take into account both tumor extent and viability of the remnant liver. Indications and contraindications for surgery vary widely and are beyond the scope of this chapter. A balanced discussion between the patient and the surgeon should address the surgical risk involved, morbidity and mortality, and the anticipated results: Disease-free and overall survival.
The underlying liver parenchyma (cirrhosis, steatosis, or normal) dictates how the patient will tolerate the surgical insult. Careful history and physical examination may help predict the status of the liver parenchyma, but unanticipated cirrhosis and steatosis are still encountered. A history of hepatitis or drug and alcohol abuse should be documented. Cachexia, jaundice, ascites, and portal hypertension are all stigmata of cirrhosis. In the setting of metastatic colorectal cancer, the long-term effects of chemotherapy may alter hepatic reserve and regeneration potential. Safe surgical interventions are clearly possible, but steatosis and steatohepatitis increase the surgical risk. Despite a careful history and physical, unrecognized liver diseases and even subclinical cirrhosis can be encountered at the time of surgery. A needle biopsy of “normal” liver may be the best way to preoperatively evaluate for subclinical liver disease. The surgical plan and operative consent should be flexible enough to accommodate the unexpected.
The volume and quality of the liver must be sufficient to sustain the patient during and after surgery. A healthy arterial and portal inflow, venous outflow, and biliary drainage are required. Judicious use of cautery, sutures and intraoperative ischemia, and Pringle maneuver (see the following section) can minimize damage to the remaining liver parenchyma. Up to 66% of the liver can be safely resected if the remnant liver is healthy. With resections involving greater than 50%, transient jaundice and ascites may develop but typically resolve within 1 to 2 months. Efforts to predict postoperative liver failure still lack sensitivity and specificity. Clinical judgment and experience are required. In the diseased liver, the minimum volume of liver required to prevent liver failure is difficult to predict. In general, the cirrhotic liver with signs of end-stage liver failure such as portal hypertension or ascites will not tolerate an anatomic liver resection and strong consideration should be given to parenchyma-sparing surgery such as wedge resections or ablative procedures.
The appropriate radiographic studies (computed tomography or magnetic resonance imaging) must be obtained before initiation of chemotherapy and carefully studied. For metastatic colorectal cancer, treatment decisions should always be based on the pretreatment imaging. A careful assessment of the extent of disease needs to be done before embarking on major hepatic surgery. This should include evaluation of the extra- and intrahepatic disease burden as well as the quality of the liver parenchyma. Peritoneal disease, extensive nodal disease (of the primary cancer or within the hepatic nodal distribution), or numerous unanticipated hepatic lesions are all relative contraindications for hepatic resection. Preoperative chemotherapy, staged procedures, portal vein embolization, and ablative techniques can increase curative options and ensure margin-negative resections.
The Couinaud system numbers the anatomic segments of the liver (Fig. 82.1). A clear understanding of segmental liver anatomy is critical when planning a hepatic resection. This system is based on portal vein and biliary anatomy. It provides safe segmental anatomy on which to base surgical resection lines. Any surgical resection can be labeled numerically; for example, a right hepatectomy corresponds to resection of segments V to VIII and left hepatectomy segments II to IV. When
segment I (caudate) is removed, this should be stipulated. Using the segmental (numerical) terminology can ensure that the radiologist, medical oncologist, and hepatic surgeon are communicating effectively.
segment I (caudate) is removed, this should be stipulated. Using the segmental (numerical) terminology can ensure that the radiologist, medical oncologist, and hepatic surgeon are communicating effectively.
This chapter provides the technical steps involved in performing a nonanatomic wedge resection, a left lateral bisegmentectomy (segments II and III), and left and right hepatectomies. These general techniques can be modified and combined as needed on the basis of tumor distribution, keeping in mind that the more extensive the resection, the higher the risk of transient or even possibly permanent liver failure (especially in the cirrhotic liver). Extended resection or trisegmentectomies will not be covered because these procedures are best performed by experienced hepatobiliary surgeons. These are covered in the references at the end of the chapter.
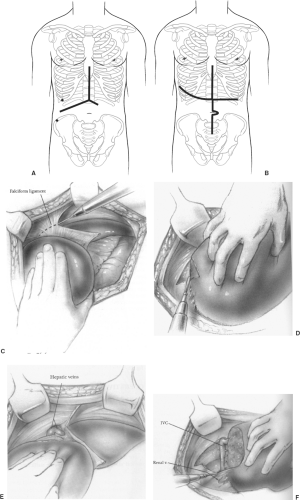
Incision and Initial Hepatic Mobilization (Fig. 82.2)
Technical Points
The liver fills the right upper quadrant of the abdomen and exposure is critical to safe surgery. A right subcostal with vertical midline extension or bilateral subcostal incision will provide wide exposure for most hepatic resections (Fig. 82.2A). A self-retaining retractor with rib elevation is helpful. Sternal split or right anterior thoracotomy is seldom required but must be kept within the surgical armamentarium for difficult situations (Fig. 82.2B). Small wedge resections or peripheral segmentectomies can be accomplished through upper midline incisions and laparoscopic mobilization and resections are now being accomplished safely.
Release the liver from supporting structures before embarking on major hepatic procedures. The falciform contains the obliterated umbilical vein and can be divided low and used for traction. Above the liver, the thin avascular ligament is divided with cautery (Fig. 82.2C). When the ligament nears the diaphragm it widens and will lead directly to the left and middle hepatic veins. The left triangular ligament can be divided laterally with cautery by gently pulling the lateral segments (II and III) downward and rotating medially. With thick or diseased livers, the lateral-most corner may be difficult to see, resulting in a lateral tear if too much traction is applied. If a lateral tear occurs, it is easily repaired after being completely released from the diaphragm. In these cases, begin medially by placing a finger or laparotomy sponge between the proximal stomach and push upward to expose the thin ligament between the diaphragm liver surfaces. Divide this medially and work laterally.
The right triangular ligament is divided by lifting and rolling the liver medially using a laparotomy pad to create gentle tension between the ligament and the diaphragm (Fig. 82.2D). Working close to the liver surface is safe because there are no major vascular structures until the right hepatic vein and retrohepatic veins are encountered entering the vena cava (Fig. 82.2E, F). At completion, the inferior vena cava can be visualized and small retrohepatic veins divided with care. For tumors invading the diaphragm, resection en bloc with the liver should be done and the diaphragm defect closed primarily. If bleeding from the diaphragm occurs, the vessels can retract into the muscular layers and should be controlled with suture ligature. After releasing the right and left triangular ligaments, the liver should be mobile within the abdomen. This is the best time to confirm the surgical plan and ensure the incision is adequate for the proposed procedure. Intraoperative ultrasound is a useful adjunct to identify lesions and ensure that planned segments to remain are disease free.
Anatomic Points
The liver is attached to the diaphragm relatively posteriorly by a series of peritoneal reflections, termed ligaments. As the peritoneal leaves of the falciform ligament reach the liver, they diverge to the left and right to form the coronary ligaments that surround the bare area of the liver. This region of the liver is described as bare because it is not covered by peritoneum. On the right, the coronary ligament consists of anterior (superior) and posterior (inferior) layers that are widely separated from each other. On the left, the anterior and posterior layers are quite close, separated from each other only by a modest amount of connective tissue. Within this connective tissue run some variable vessels, nerves, and, frequently, biliary radicles. The left triangular ligament forms the upper boundary of the superior recess of the omental bursa, whereas the superior layer of the right coronary ligament prevents the manual exploration of the diaphragmatic surface of the liver. Division of the coronary ligament and the right or left triangular ligament, or both, is necessary to mobilize the liver and expose the hepatic part of the inferior vena cava. The coronary and right triangular ligaments are simply peritoneal reflections and can be sharply divided with no special precautions. The long, narrow left triangular ligament always contains vessels or bile canaliculi, or both, and thus should be divided between the clamps. As the incision of these peritoneal reflections progresses medially, the hepatic veins will begin to appear.
Inflow Control (Pringle Maneuver) (Fig. 82.3)
Technical Points
Control of vascular inflow to the liver should be in the armamentarium of all general surgeons (Pringle maneuver). A medial to lateral mobilization of the porta hepatis is fast and safe. Begin
by lifting the left lateral liver (segments II and III) to expose the caudate lobe (segment I) and divide the lesser omentum (avascular tissue between the lesser curve of the stomach and liver). A finger placed directly on the caudate lobe can be swept to the right (above the inferior vena cava) to encircle the porta hepatis through the foramen of Winslow. Circumferential control of the hepatic artery and portal vein can be done without any dissection or mobilization. Palpation of the porta hepatis can define the right and left portal veins and hepatic arterial divisions. Arterial anomalous patterns are common. A left hepatic artery directly off the celiac axis or right hepatic arteries off the superior mesenteric artery are frequently encountered.
by lifting the left lateral liver (segments II and III) to expose the caudate lobe (segment I) and divide the lesser omentum (avascular tissue between the lesser curve of the stomach and liver). A finger placed directly on the caudate lobe can be swept to the right (above the inferior vena cava) to encircle the porta hepatis through the foramen of Winslow. Circumferential control of the hepatic artery and portal vein can be done without any dissection or mobilization. Palpation of the porta hepatis can define the right and left portal veins and hepatic arterial divisions. Arterial anomalous patterns are common. A left hepatic artery directly off the celiac axis or right hepatic arteries off the superior mesenteric artery are frequently encountered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree