Lymphocytosis
Qian-Yun Zhang, MD, PhD
Key Facts
Etiology/Pathogenesis
Lymphocytosis in children most likely benign
Persistent lymphocytosis in adults warrants diligent work-up to exclude neoplastic process
More than 90% of humans are infected by EBV
EBV infects B lymphocytes and becomes latent; individual becomes lifelong EBV carrier
Persistent polyclonal B-cell lymphocytosis may show underlying genetic abnormality
Transient stress lymphocytosis is likely induced by epinephrine released by body or administered iatrogenically
It is difficult to distinguish reactive from clonal lymphoproliferative disorders in large granular lymphocytosis
Clinical Issues
Flow cytometric studies should be performed on all persistent lymphocytosis in adults
Assessment of T-cell receptor gene rearrangement by PCR or Southern blot is helpful to assess clonality
Microscopic Pathology
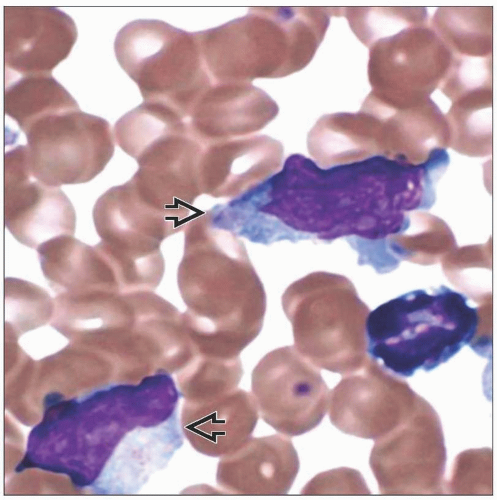
PB of IM reveals Downey type II or III lymphocytes
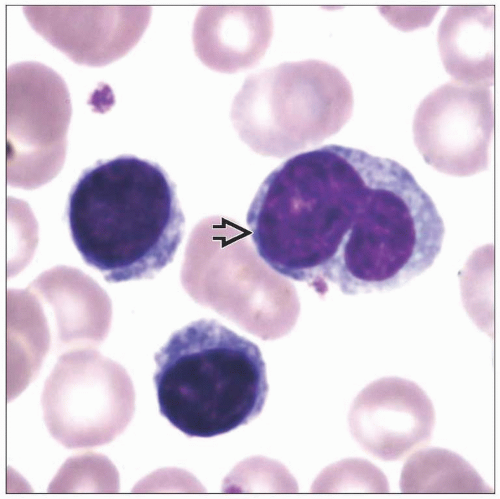
Persistent polyclonal B-cell lymphocytosis shows mature nonreactive lymphocytes with frequent bilobed nuclei
Transient stress lymphocytosis shows mature nonreactive lymphocytes
Large granular lymphocytes have abundant pale cytoplasm and azurophilic granules
TERMINOLOGY
Synonyms
Reactive lymphocytosis
Definitions
Elevated blood lymphocyte count above age-defined normal range
ETIOLOGY/PATHOGENESIS
Etiology
Lymphocytosis with reactive morphology
Viral infections, such as EBV, CMV, or other
Lymphocytosis with nonreactive morphology
Whooping cough (pertussis)
Transient stress lymphocytosis (TSL)
Persistent polyclonal B-cell lymphocytosis (PPBL)
Large granular lymphocytosis
T-cell large granular or chronic NK-cell lymphocytosis
Other causes of lymphocytosis
Neoplasia, postsplenectomy, or hypersensitive reactions
Pathogenesis
Infectious mononucleosis (IM)
EBV is DNA virus: Member of Herpesviridae family
EBV infects B lymphocytes and becomes latent; individual becomes lifelong EBV carrier
Acute infection induces cytotoxic T-cell response that eventually curbs viral proliferation and restrains immune response
Rarely chronic active EBV infection (CAEBV) develops; leads to progressive immunodeficiency
Hemophagocytic syndrome (HPS) and lymphoproliferative disorders can develop in CAEBV
In fatal IM, lymphocytes invade vital organs, cause HPS, massive marrow necrosis, and severe pancytopenia
Monoclonal or biclonal EBV and immunoglobulin heavy chain (IgH) or T-cell receptor (TCR) gene rearrangements are common in fatal IM
Nonfatal IM usually lacks monoclonal EBV, IgH@, or TRG@
Fatal IM patients are usually immunocompromised either by inherited immunodeficiency disorder, from immunosuppressants, or HIV infection
Persistent polyclonal B-cell lymphocytosis (PPBL)
Possible underlying genetic abnormality and familial occurrence
Association with HLA DR7 phenotype in some cases
Frequent detection of BCL2-IGH@ gene rearrangements and tri(3)(q10) in patients and first-degree relatives
Inhibition of apoptosis is proposed as cause for accumulation of peripheral blood lymphocytes
Transient stress lymphocytosis (TSL)
Associated with acute stressor, such as trauma, myocardial infarct, obstetric emergencies, sickle cell crisis, septic shock, status epilepticus
Likely induced by epinephrine released by body or administered iatrogenically
Large granular lymphocytosis
Difficult to distinguish reactive from clonal lymphoproliferative disorders
T-cell large granular lymphocytosis
Associated with solid tumor, lymphomas, viral infections, immune-mediated thrombocytopenia, or hemophagocytosis
Pathogenesis unclear; expansion in response to antigen stimulation possible
Chronic NK-cell lymphocytosis
CLINICAL ISSUES
Epidemiology and Clinical Presentation
Infectious mononucleosis (IM)
More than 90% of humans are infected
Most common pattern of EBV infection is clinically silent, childhood infection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree