Low Anterior Rectal Resection: Robotic-Assisted Laparoscopic Technique
Mehraneh D. Jafari
Alessio Pigazzi
DEFINITION
Low anterior resection (LAR) is most commonly performed for patients with mid to low non-sphincter-invading rectal adenocarcinoma. A simple surgical definition of LAR is full mobilization and division of the rectum at the level of the levators, leaving behind only a short or no rectal stump.
LAR for rectal cancer requires a total mesorectal excision (TME) to ensure a radical resection with adequate radial margins.1 The goal is to achieve an en bloc resection of the cancer with complete dissection of the pararectal lymph nodes contained within the mesorectum.
Robotic-assisted laparoscopic LAR is a novel surgical technique that allows for a minimally invasive approach to TME. Robotic LAR can be performed via totally robotic or laparoscopic/robotic hybrid techniques. Our preferred method is a hybrid approach involving a laparoscopic medial to lateral mobilization of the colon and of the splenic flexure followed by a robotic TME.
PATIENT HISTORY AND PHYSICAL FINDINGS
A full history and physical examination will allow the surgeon to determine if a sphincter-sparing operation is possible, whether a temporary ileostomy is likely, and will also aid in discussions regarding postoperative functional status.
History elements elicited should include baseline functional status, bowel incontinence, sexual dysfunction, urinary dysfunction as well as pain with defecation or tenesmus. Previous history of pelvic radiation and pelvic surgery should also be noted.
History of incontinence should prompt discussions regarding postoperative quality of life with a low anastomosis.
History of pain or tenesmus suggests involvement of the anal sphincter or a larger tumor. This will alter the course of treatment, and a sphincter-sparing operation may not be possible in this subgroup of patients.
Physical examination should include a digital rectal examination (DRE), vaginal examination, anoscopy, and a thorough abdominal examination.
DRE should assess tumor size, degree of fixation to rectal and pelvic wall, mobility, location (anterior/posterior/lateral), distance from the anorectal ring, and anterior extension into vagina/prostate. Anal sphincter involvement can also be determined by DRE in the majority of patients.
Anterior rectal tumors in female patients require a vaginal examination to rule out extension into the vagina.
Anoscopy for low rectal tumors may allow for better visualization of the tumor during the physical examination.
The abdominal examination should evaluate for liver metastasis. A bilateral groin examination should be performed to evaluate for potential inguinal lymphadenopathy.
IMAGING AND OTHER DIAGNOSTIC STUDIES
The physical examination in conjunction with endoscopy and imaging modalities will aid in the preoperative surgical evaluation and staging. This preoperative workup will dictate the best surgical approach, the need for temporary diversion, and the need for neoadjuvant therapy.
Colonoscopy must be performed in all patients with rectal cancer.
This will allow for assessment of tumor location and pathology.
It will also serve to rule out and possibly remove any synchronous colonic lesions. Malignant synchronous lesions have been reported in 2% to 8% of cases and benign synchronous polyps in 13% to 62% of cases.2, 3, 4
If a colonoscopy has already been done by another provider, it is our preference to perform a flexible sigmoidoscopy in all patients for documentation of the size, location, and distance of the tumor from the anal sphincter complex.
The use of preoperative tattoos in rectal cancer patients undergoing anterior resection is unnecessary and unreliable to determine distal margins. The best assessment of the margin is obtained via frequent and thorough digital examinations, intraoperative flexible endoscopy, and adherence to the best TME surgery criteria.
Accurate staging of rectal cancer should be able to determine depth of invasion, presence of lymph node metastases, and resectability of locally advanced tumors.
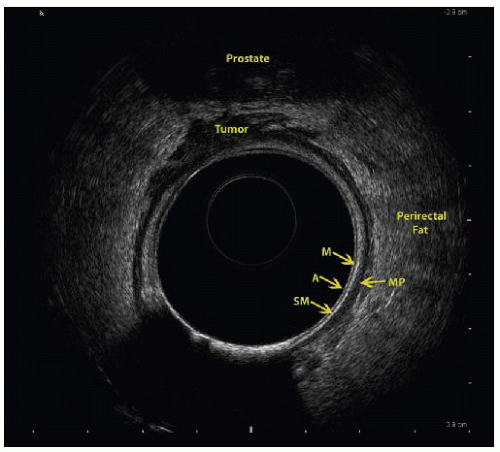
Endorectal ultrasound has an overall 80% to 95% staging accuracy.5
The ability to visualize the layers of the bowel wall allows for accurate T staging (FIG 1).
T1 stage is associated with 88% sensitivity and 98% specificity.
T2 stage is associated with 81% sensitivity and 96% specificity.
T3 stage is associated with 96% sensitivity and 91% specificity.
T4 stage is associated with 95% sensitivity and 98% specificity.5
Detection of lymph node metastasis is associated with 73% sensitivity and 76% specificity.6
High-resolution pelvic magnetic resonance imaging (MRI) delineates the layers of the bowel wall in T2 weighted images. It is associated with 93% to 97% sensitivity for T staging and 77% sensitivity for lymph node metastasis.7,8
SURGICAL MANAGEMENT
Preoperative Planning
Surgical decision is based on rectal cancer staging. As per NCCN guidelines, neoadjuvant chemotherapy and radiation therapy (CRT) should be considered for all N+ positive tumors based on preoperative imaging. The use of neoadjuvant CRT in T3N0 tumors is somewhat controversial. Proximal T3 tumors with no involvement of the circumferential resection margin (i.e., posterior lesions surrounded by abundant mesorectum) can selectively undergo radical resection without CRT.9
Neoadjuvant CRT has been shown to reduce the local recurrence rate and increase the chances of sphincter-sparing surgery.9
The decision for neoadjuvant chemotherapy should stem from a multidisciplinary discussion amongst the surgeon, oncologist, radiation oncologist, and patient.
An enterostomal therapist should be involved for counseling and for potential stomal marking prior to operation.
Despite the debate regarding bowel preparation, we routinely use mechanical bowel preparation at our institution for easier manipulation of the bowel during surgery. Our institution’s standard bowel preparation is 510 mg of MiraLAX® in 128 oz of Gatorade®.
Rectal irrigation via saline solution is performed in all patients.
A Foley catheter is placed in all patients after induction for bladder decompression.
Prophylactic ertapenem (Invanz®) antibiotic is administered prior to induction of anesthesia.
Positioning
The patient is placed in a modified lithotomy position with attention placed to correct technique to minimize injury:
The patient is ideally placed on a large high-density viscoelastic foam mat to prevent sliding.
The patient is brought to the edge of the table and the legs are placed into Yellofin® or Allen® stirrups with the hips slightly flexed and abducted, the feet flat within the stirrups, and pressure avoided along the lateral aspects of the legs. The ankle, knee, and contralateral shoulder should be aligned.
A Velcro belt is strapped over the chest to prevent side-to-side sliding.
The perineum is prepped if a transanal extraction and or hand-sewn anastomosis is anticipated.
TECHNIQUES
LAPAROSCOPIC MEDIAL TO LATERAL DISSECTION OF COLON
Port Placement
Pneumoperitoneum is established via a Veress needle at Palmer’s point (1 to 2 cm below the left costal border in the midclavicular line [MCL]).
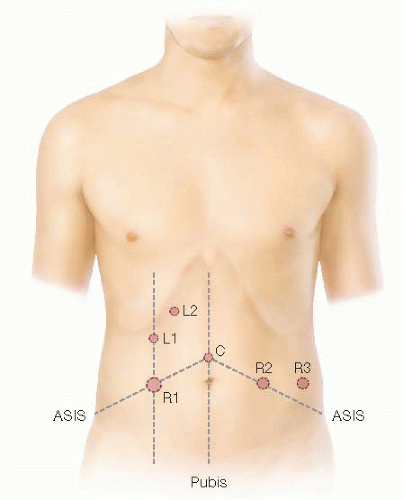
The ports are triangulated and placed at a minimum of one handbreadth apart (FIG 2).
The camera (C) port is placed halfway between the xiphoid process and symphysis pubis.
Three robotic (R) ports will be placed as follows (FIG 2):
R1 is a 12-mm trocar inserted in the MCL halfway in between C and the right anterior superior iliac spine (ASIS). This port can be used for ileostomy placement at the end of the surgery.
R2 is an 8-mm trocar inserted as a mirror image of R1.
R3 is an 8-mm trocar inserted 8 to 10 cm lateral to R2, usually directly above the left ASIS.
Laparoscopic-assisted (L) ports (FIG 2):
L1 is a 5-mm trocar inserted in the MCL about 12 cm superior to R1.
L2 is a 5-mm port inserted halfway between MCL and midline about 12 cm superior to L1.
Both surgeon and assistant stand on the right side of patient (FIG 3).
R1, L1, L2, and C ports are used during the laparoscopic section.
Laparoscopic Transection of the Inferior Mesenteric Vein
The peritoneal cavity is explored for evidence of metastatic disease.
The patient is placed in a Trendelenburg position with the left side elevated.
The small bowel is swept out of the pelvis. Nontraumatic bowel graspers are used to avoid injury.
The dissection is begun at the inferior mesenteric vein (IMV), lateral to the ligament of Treitz (FIG 4).
The IMV is identified and dissected from its attachments to the left mesocolon.
The peritoneum is scored with monopolar electrocautery.
Blunt dissection is used to skeletonize the vessel. Once this is achieved, the vessel is clipped and divided via vessel sealer device just below the pancreas. This can also be accomplished with an Endo GIA vascular stapler.
Transection of the IMV will serve as a lengthening maneuver, which in turn will decrease tension on the anastomosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree