Introduction
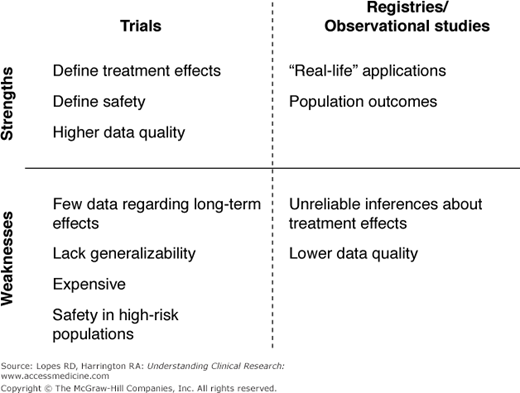
The imperative for sound clinical research continues to increase in response to the demand for evidence-based, guideline-driven medicine. To increase the quality of health care and improve clinical decision-making, innumerable studies have contributed to a hierarchy of evidence for each disease state. The randomized trial often stands at the pinnacle of this hierarchy, yielding the highest level of evidence through a blinded experiment of an intervention or treatment. Preceding observational studies often generate the equipoise and hypotheses for randomized trials. In other instances, observational research can be the best vehicle for generating the evidence. The experimentation inherent in a randomized trial might be unnecessary, inappropriate, or even impossible under certain circumstances. Political, legal, and ethical barriers also can prohibit randomized trials. Increasingly, the costs of large trials are creating another barrier to generating randomized evidence, reducing incentives for funding the head-to-head trials needed to understand the real-world effectiveness of novel therapies. It is therefore important, in the context of the emerging field of comparative effectiveness research, to understand the value and appropriateness of observational research versus randomized trials in answering different clinical questions (Figure 16–1). Prior observational studies, both successes and failures, can inform future efforts in comparative effectiveness.
Value of Observational Research in the Hierarchy of Evidence
Hierarchies of evidence are not the same across disease states. In prevalent conditions with substantial morbidity and mortality, public and private resources are often devoted to generating high-quality evidence from randomized trials. However, even in cardiovascular disease, the leading cause of death in the United States, guideline recommendations are largely developed from lower-level sources of evidence or expert opinion (1,2).
In unstudied subgroups, observational data often serve as guidance for clinical decision-making. For example, age is a significant determinant of outcomes for patients with acute coronary syndromes (ACS), and almost 25% of percutaneous coronary interventions (PCIs) in contemporary practice are performed in patients older than 75 years (3,4). However, most clinical trials exclude such patients. From 1991 to 2000, patients older than 75 years accounted for only 9% of trial enrollment, despite accounting for 37% of all patients with myocardial infarction (MI) in the United States (5). In place of randomized clinical trials, large clinical registries have provided much of the information that informs treatment patterns for this growing subgroup of patients undergoing coronary revascularization.
The American Heart Association (AHA) 2007 scientific statement on acute coronary care in the elderly (6) relied on the National Registry of Myocardial Infarction (NRMI), the Global Registry of Acute Coronary Events (GRACE) (7), and the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines? (CRUSADE) national quality improvement initiative (8) to determine in-hospital and long-term outcomes for elderly patients (Table 16–1) (6). Complementing data from randomized trials (9–13), these registries indicated that elderly patients with ACS are at high risk for death and other adverse events and therefore derive greater benefit from revascularization than do younger patients. Observational data from these registries will continue to complement trial data by monitoring the safety and efficacy of ACS therapies in the elderly to better understand their utility in this understudied subgroup.
Source | Enrollment years | n | Age ≥75 years (%) | Regions | Randomized treatment(s) |
|---|---|---|---|---|---|
VIGOUR (pooled) | |||||
GUSTO-IIb (9) PARAGON-A (10) PARAGON-B (11) PURSUIT (12) GUSTO IV-ACS (13) | 1994–1996 1995–1995 1997–1999 1995–1997 1998–2000 | 8011 2282 5225 10,948 7800 | 19.5 19.1 17.8 14.6 22.7 | 9 countries 20 countries 26 countries 28 countries 24 countries | Hirudin vs. heparin GPI (lamifiban) vs. heparin GPI (lamifiban) vs. placebo GPI (eptifibatide) vs. placebo GPI (abciximab) vs. placebo |
NRMI 2–4 (6) | 1994–2003 | 10,76,796 | 38.3 | United States | NSTE MI registry |
GRACE (7) | 1999–2004 | 11,968 | 31.6 | 14 countries | NSTE ACS registry |
CRUSADE (8) | 2001–2003 | 56,963 | 39.9 | United States | NSTE ACS QI initiative |
Discrepancies between Observational Research and Randomized Trials
The results of clinical trials often diverge from observational data. For example, prior observational studies established total plasma homocysteine level as an independent cardiovascular risk factor (14–18). These findings were disproven by subsequent randomized studies, however, which showed that lowering homocysteine levels had no effect on recurrent cardiovascular disease in patients with acute MI or on major cardiovascular events in patients with vascular disease (19,20). Similarly, observational data suggested that vitamin E supplementation was associated with a reduced risk of coronary artery disease (CAD) and progression of coronary artery lesions (21–23). Subsequent randomized data indicated that long-term vitamin E supplementation did not affect cardiovascular outcomes (24).
The discrepancy between observational research and randomized trials is well illustrated by the case of hormone replacement therapy (HRT). After first receiving approval for the treatment of menopausal symptoms, HRT subsequently was found in numerous observational studies to have potentially beneficial effects in a variety of conditions. A 1992 meta-analysis in the Annals of Internal Medicine (25) recapitulated many of the observed benefits in observational studies, concluding that HRT should probably be recommended for women who have had a hysterectomy, who have CAD, or who are at high risk for CAD. Several meta-analyses, including the 1992 report, drew from the Nurses’ Health Study, in which 12,1700 female nurses aged 30–55 years completed mailed questionnaires about postmenopausal hormone use and medical history (26). The study estimated the relative risk (RR) of CAD with the use of HRT at 0.61 overall (95% confidence interval [CI], 0.52–0.71), with an RR of 0.55 (95% CI, 0.45–0.68) for women taking estrogen alone and 0.64 (95% CI, 0.49–0.85) for women taking estrogen plus progestin.