Abbreviations and Acronyms
| Ab | Antibody |
| Abn | Abnormal |
| AFB | Acid-fast bacillus |
| Ag | Antigen |
| AIDS | Acquired immunodeficiency syndrome |
| ALT | Alanine aminotransferase |
| ANA | Antinuclear antibody |
| AST | Aspartate aminotransferase |
| CBC | Complete blood cell count |
| CF | Complement fixation |
| CHF | Congestive heart failure |
| CIE | Counterimmunoelectrophoresis |
| CK | Creatine kinase |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CXR | Chest x-ray |
| CYP | Cytochrome P450 |
| Diff | Differential cell count |
| EDTA | Ethylenediaminetetraacetic acid (edetate) |
| ELISA | Enzyme-linked immunosorbent assay |
| GI | Gastrointestinal |
| GNR | Gram-negative rod |
| GNCB | Gram-negative coccobacillus |
| GPC | Gram-positive coccus |
| GVCB | Gram-variable coccobacillus |
| HLA | Human leukocyte antigen |
| Ig | Immunoglobulin |
| IM | Intramuscular(ly) |
| INR | International Normalized Ratio |
| IV | Intravenous(ly) |
| Min | Minute |
| MN | Mononuclear cell |
| MRI | Magnetic resonance imaging |
| N | Normal |
| Neg | Negative |
| NPO | Nothing by mouth (nil per os) |
| PCR | Polymerase chain reaction |
| PMN | Polymorphonuclear neutrophil (leukocyte) |
| PO | Orally (per os) |
| Pos | Positive |
| PTH | Parathyroid hormone |
| RBC | Red blood cell |
| RPR | Rapid plasma reagin (syphilis test) |
| SIADH | Syndrome of inappropriate antidiuretic hormone (secretion) |
| SLE | Systemic lupus erythematosus |
| T3 | Triiodothyronine |
| T4 | Tetraiodothyronine (thyroxine) |
| TSH | Thyroid-stimulating hormone |
| V | Variable |
| VDRL | Venereal Disease Research Laboratory (syphilis test) |
| WBC | White blood cell |
| Wk | Week |
| Yr | Year |
| ↑ | Increased |
| ↓ | Decreased |
| ↔ | No change |
How to Use This Section
This section contains information about commonly used laboratory tests. It includes most of the blood, urine, and cerebrospinal fluid tests found in this book, with the exception of drug levels and pharmacogenetic tests (see Chapter 4). Entries are in outline format and are arranged alphabetically.
This first outline listing begins with the common test name, the specimen analyzed, and any test name abbreviation (in parentheses).
Below this, in the first outline listing, is the reference range (also called reference interval) for each test. The first entry is in conventional units, and the second entry (in [brackets]) is in SI units (Système International d’Unités). Any panic values for a particular test are placed here after the word Panic. The reference ranges provided are from several large medical centers; consult your own clinical laboratory for those used in your institution.
This outline listing also shows which tube to use for collecting blood and other body fluids, how much the test costs (in relative symbolism; see below), and how to collect the specimen.
The scale used for the cost of each test is:
| Approximate Cost | Symbol Used in Tables |
|---|---|
| $1–20 | $ |
| $21–50 | $$ |
| $51–100 | $$$ |
| > $100 | <SPAN role=presentation tabIndex=0 id=MathJax-Element-1-Frame class=MathJax style="POSITION: relative" data-mathml='‘> |
Listed below are the common collection tubes and their contents:
| Tube Top Color | Tube Contents | Typically Used In |
|---|---|---|
| Lavender or Pink | K2EDTA | Complete blood count; blood banking (plasma); molecular testing (cell-based) |
| Serum separator tube (SST) | Clot activator and serum separator gel | Serum chemistry tests |
| Red | None | Blood banking (serum) |
| Blue | Sodium citrate | Coagulation studies |
| Green | Sodium heparin or lithium heparin | Plasma chemistry tests; chromosome analysis (sodium heparin) |
| Yellow | Acid citrate dextrose (ACD); sodium polyanethol sulfonate (SPS) | ACD: HLA typing; blood banking (plasma); flow cytometry SPS: Blood culture (microbiology) |
| Navy | Trace metal-free | Trace metals (eg, lead, mercury, arsenic) |
| Gray | Inhibitor of glycolysis (sodium fluoride) | Lactic acid; glucose |
| Plasma preparation tube (PPT) | K2EDTA and separator gel | Molecular testing (plasma-based), plasma chemistry tests |
This outline listing contains physiologic information about the substance being tested. Information on classification and biologic importance, as well as interactions with other biologic substances and processes, is included.
This outline lists clinical conditions that affect the substance being tested. Generally, conditions with higher prevalence are listed first. When the sensitivity of the test for a particular disease is known, that information follows the disease name in parentheses, for example, “rheumatoid arthritis (83%).” Some of the common drugs that can affect the test substance in vivo are also included in this outline listing.
ABO Typing
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
ABO typing, serum or plasma and red cells (ABO) Red or lavender/pink $ Properly identified and labeled blood specimens are critical. | The ABO antigen and antibodies remain the most significant for transfusion practice. The 4 blood groups A, B, O, and AB are determined by the presence of antigens A and B or their absence (O) on a patient’s red blood cells. Individuals possess antibodies directed toward the A or B antigen absent from their own red cells. In the US white population, 45% are type O, 40% A, 11% B, 4% AB. In the US Hispanic population, 57% are type O, 30% A, 10% B, 3% AB. In the African American population, 49% are type O, 27% A, 20% B, 4% AB. In the US Asian population, 40% are type O, 28% A, 27% B, 5% AB. In the Native American population, 55% are type O, 35% A, 8% B, 2% AB. | Type O patients can receive type O red cells and type A, B, O, or AB plasma. Type A patients can receive type A or O red cells and type A or AB plasma. Type B patients can receive type B or O red cells and type B or AB plasma. Type AB patients can receive type AB, A, B, or O red cells but only type AB plasma. In an emergency situation, type O red cells and type AB plasma may be given to patients with any ABO blood types. | For both blood donors and recipients, routine ABO typing includes both red cell and serum testing, as checks on each other. Tube testing is as follows: patient’s red cells are tested with anti-A and anti-B for the presence or absence of agglutination (forward or cell type), and patient’s serum or plasma is tested against known A and B cells (reverse or serum/plasma type). Technical Manual of the American Association of Blood Banks, 17th ed. American Association of Blood Banks, 2011. Malomgré W et al. Recent and future trends in blood group typing. Anal Bioanal Chem 2009;393:1443. [PubMed: 18839152] |
Acetaminophen
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Acetaminophen, serum (Tylenol; others) 10–20 mg/L [66–132 mcmol/L] Panic: >50 mg/L Red $$ For suspected overdose, draw 2 samples at least 4 hours apart, at least 4 hours after ingestion. Note time of ingestion, if known. Order test stat. | In overdose, liver and renal toxicity are produced by the hydroxylated metabolite if it is not conjugated with glutathione in the liver. | Increased in: Acetaminophen overdose. Interpretation of serum acetaminophen level depends on time since ingestion. Levels drawn < 4 hr after ingestion cannot be interpreted, since the drug is still in the absorption and distribution phase. Use nomogram (Figure 10–1) to evaluate possible toxicity. Levels >150 mg/L at 4 hours or >50 mg/L at 12 hours after ingestion suggest toxicity. Nomogram is inaccurate for chronic ingestions. | Do not delay acetylcysteine (Mucomyst) treatment (140 mg/kg orally) if stat levels are unavailable. Chun LJ et al. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol 2009;43:342. [PubMed: 19169150] Green TJ et al. When do the aminotransferases rise after acute acetaminophen overdose? Clin Toxicol (Phila) 2010;48:787. [PubMed: 20969501] Klein-Schwartz W et al. Intravenous acetylcysteine for the treatment of acetaminophen overdose. Expert Opin Pharmacother 2011;12:119. [PubMed: 21126198] |
Acetoacetate
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Acetoacetate, blood or urine 0 mg/dL [mcmol/L] Red, lavender, or urine container $ Urine sample should be fresh. | Acetoacetate, acetone, and β-hydroxybutyrate contribute to ketoacidosis when oxidative hepatic metabolism of fatty acids is impaired. Proportions in serum vary but are generally 20% acetoacetate, 78% β-hydroxybutyrate, and 2% acetone. | Present in: Diabetic ketoacidosis, alcoholic ketoacidosis, prolonged fasting, starvation, severe carbohydrate restriction with normal fat intake, prolonged exercise. | Nitroprusside test is semiquantitative; it detects acetoacetate and is sensitive down to 5–10 mg/dL. Trace = 5 mg/dL, small = 15 mg/dL, moderate = 40 mg/dL, large = 80 mg/dL [1 mg/dL = 100 mcmol/L]. β-Hydroxybutyrate has no ketone group; therefore, it is not detected by the nitroprusside test. Acetone is also not reliably detected by this method because the sensitivity for acetone is poor. Failure of nitroprusside test to detect β-hydroxybutyrate in ketoacidosis may produce a seemingly paradoxical increase in ketones with clinical improvement as nondetectable β-hydroxybutyrate is replaced by detectable acetoacetate. Testing for blood β-hydroxybutyrate is clinically more useful in this setting. Prisco F et al. Blood ketone bodies in patients with recent-onset type 1 diabetes (a multicenter study). Pediatr Diabetes 2006;7:223. [PubMed: 16911010] Weber C et al. Prevention of diabetic ketoacidosis and self-monitoring of ketone bodies: an overview. Curr Med Res Opin 2009;25:1197. [PubMed: 19327102] |
Acetylcholine Receptor Antibody
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Acetylcholine receptor antibody, serum Negative SST, red $$ | Acetylcholine receptor antibodies are involved in the pathogenesis of myasthenia gravis. Sensitive radioimmunoassay or enzyme-linked immunosorbent assay (ELISA) is available based on inhibition of binding of 125I α-bungarotoxin to the acetylcholine receptor. | Positive in: Myasthenia gravis (sensitivity 87–98%, specificity 98–100%) | Antibody levels correlate with severity of autonomic failure. Antibodies can also be associated with other neurologic disorders unrelated to the autonomic nervous system. Leite MI et al. Diagnostic use of autoantibodies in myasthenia gravis. Autoimmunity 2010;43:371. [PubMed: 20380582] Meriggioli MN. Myasthenia gravis with anti-acetylcholine receptor antibodies. Front Neurol Neurosci 2009;26:94. [PubMed: 19349707] Meriggioli MN et al. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 2009;8:475. [PubMed: 19375665] Winston N et al. Recent advances in autoimmune autonomic ganglionopathy. Curr Opin Neurol 2010;23:514. [PubMed: 20634694] |
Activated Clotting Time
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Activated clotting time, whole blood (ACT) 70–180 sec (method-specific) $$ Obtain blood in a plastic syringe without anticoagulant. Test should be performed immediately at patient’s bedside. A clean venipuncture is required. A special vacutainer tube containing activator (eg, celite, kaolin) is also available. | ACT is a point-of-care test used to monitor high-dose heparin as an anticoagulant during cardiac surgery (extracorporeal circulation), angioplasty, and hemodialysis. It is also used to determine the dose of protamine sulfate to reverse the heparin effect on completion of the procedure. ACT is also used to monitor heparin or direct thrombin inhibitor in patients with documented lupus anticoagulant. | Prolonged in: Heparin therapy, direct thrombin inhibitor therapy, severe deficiency of clotting factors (except factors VII and XIII), functional platelet disorders. In general, the accepted goal during cardiopulmonary bypass surgery is 400–500 sec. For carotid artery stenting, the optimal ACT is 250–300 sec. | ACT is the choice of test when heparin levels are too high (eg, > 1.0 U/mL heparin) to allow monitoring with PTT and/or when a rapid result is necessary to monitor treatment. Because different methodologies and a number of variables (eg, platelet count and function, hypothermia, hemodilution, and certain drugs like aprotinin) may affect the ACT, the ACT test is not yet standardized. Reproducibility of prolonged ACTs may be poor. Bosch YP et al. Comparison of ACT point-of-care measurements: repeatability and agreement. Perfusion 2006;21:27. [PubMed: 16485696] Perry DJ et al. Point-of-care testing in haemostasis. Br J Haematol 2010;150:501. [PubMed: 20618331] Saw J et al. Evaluating the optimal activated clotting time during carotid artery stenting. Am J Cardiol 2006;97:1657. [PubMed: 16728233] |
Adrenocorticotropic Hormone
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Adrenocorticotropic hormone, plasma (ACTH) 9–52 pg/mL [2–11 pmol/L] Lavender, pink <SPAN role=presentation tabIndex=0 id=MathJax-Element-2-Frame class=MathJax style="POSITION: relative" data-mathml='‘> Separate plasma from cells and freeze ASAP Send promptly to laboratory on ice. ACTH is unstable in plasma, is inactivated at room temperature, and adheres strongly to glass. Avoid all contact with glass. | Pituitary ACTH (release stimulated by hypothalamic corticotropin-releasing factor) stimulates cortisol release from the adrenal gland. There is feedback regulation of the system by cortisol. ACTH is secreted episodically and shows circadian variation, with highest levels at 6:00–8:00 AM; lowest levels at 9:00–10:00 PM. | Increased in: Pituitary (40–200 pg/mL) and ectopic (200–71,000 pg/mL) Cushing syndrome, primary adrenal insufficiency (> 250 pg/mL), adrenogenital syndrome with impaired cortisol production. Decreased in: Adrenal Cushing syndrome (< 20 pg/mL), pituitary ACTH (secondary adrenal) insufficiency (< 50 pg/mL). | ACTH levels can be interpreted only when measured with cortisol after standardized stimulation or suppression tests (see Adrenocortical insufficiency algorithm, Figure 9–3, and Cushing syndrome algorithm, Figure 9–8). Findling JW et al. Cushing’s syndrome: important issues in diagnosis and management. J Clin Endocrinol Metab 2006;91:3746. [PubMed: 16868050] Neary N et al. Adrenal insufficiency: etiology, diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes 2010;17:217. [PubMed: 20375886] Pecori Giraldi F. Recent challenges in the diagnosis of Cushing’s syndrome. Horm Res 2009;71(Suppl 1):123. [PubMed: 19153521] |
Alanine Aminotransferase
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Alanine aminotransferase, serum or plasma (ALT, SGPT, GPT) 0–35 U/L [0–0.58 mckat/L] (laboratory-specific) SST, PPT, green, lavender $ | Intracellular enzyme involved in amino acid metabolism. Present in large concentrations in liver, kidney; in smaller amounts, in skeletal muscle and heart. Released with tissue damage, particularly liver injury. | Increased in: Acute viral hepatitis (ALT > AST), biliary tract obstruction (cholangitis, choledocholithiasis), alcoholic hepatitis and cirrhosis (AST > ALT), liver abscess, metastatic or primary liver cancer; nonalcoholic steatohepatitis; right heart failure, ischemia or hypoxia, injury to liver (“shock liver”), extensive trauma; drugs that cause cholestasis or hepatotoxicity. Decreased in: Pyridoxine (vitamin B6) deficiency. | ALT is the preferred enzyme for evaluation of liver injury. Screening ALT in low-risk populations has a low (12%) positive predictive value and is not recommended. See Liver function tests (Table 8–14). Fraser A et al. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care 2009;32:741. [PubMed: 19131466] McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009;49(5 Suppl):S45. [PubMed: 19399792] St George A et al. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol 2009;24:399. [PubMed: 19067776] |
Albumin
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Albumin, serum or plasma 3.4–4.7 g/dL [34–47 g/L] SST, PPT, green $ | Major component of plasma proteins; influenced by nutritional state, hepatic function, renal function, and various diseases. Major binding protein. Although there are more than 50 different genetic variants (alloalbumins), only occasionally does a mutation cause abnormal binding (eg, in familial dysalbuminemic hyperthyroxinemia). | Increased in: Dehydration, shock, hemoconcentration. Decreased in: Decreased hepatic synthesis (chronic liver disease, malnutrition, malabsorption, malignancy, congenital analbuminemia [rare]). Increased losses (nephrotic syndrome, burns, trauma, hemorrhage with fluid replacement, fistulas, enteropathy, acute or chronic glomerulonephritis). Hemodilution (pregnancy, CHF). Drugs: estrogens. | Serum albumin indicates severity in chronic liver disease. Useful in nutritional assessment if there is no impairment in production or increased loss of albumin. Independent risk factor for all-cause mortality in the elderly (age >70) and for complications in hospitalized and post-surgical patients. There is a 10% reduction in serum albumin level in late pregnancy (related to hemodilution). See liver function tests (Table 8–14) and MELD scoring systems for staging cirrhosis (Table 8–9). Ghany MG et al. HALT-C Trial Group. Predicting clinical and histologic outcomes based on standard laboratory tests in advanced chronic hepatitis C. Gastroenterology 2010;138:136. [PubMed: 19766643] Hennessey DB et al. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg 2010;252:325. [PubMed: 20647925] Pencharz PB. Assessment of protein nutritional status in children. Pediatr Blood Cancer 2008;50(2 Suppl):445. |
Albumin, urine <30 mg/24 hr <20 mcg/min (timed collection) <SPAN role=presentation tabIndex=0 id=MathJax-Element-3-Frame class=MathJax style="POSITION: relative" data-mathml='‘> | The normal urinary albumin excretion is less than 30 mg/24 hr. On random spot urine collection, the albumin-to-creatinine ratio (ACR, mcg/mg) should be less than 30. The term microalbuminuria is defined as a subtle increase in the urinary excretion of albumin that cannot be detected by conventional urinalysis. Specifically, the excretion of 30–300 mg albumin per 24 hours or an ACR of 30–300 (mcg/mg) is considered microalbuminuria (urine albumin is high). 300 mg or more of albumin excretion per day or an ACR of 300 or higher indicates gross albuminuria (urine albumin very high or nephrotic) range. | Increased in: Diabetes mellitus, diabetic nephropathy. | Microalbuminuria is a useful indicator of early nephropathy in diabetic patients. Urine albumin measurement requires a sensitive immunochemical assay. Urine dipstick analysis is often insensitive to microalbuminuria. Screening for microalbuminuria is often performed by measurement of the ACR in a random spot collection (preferred method). Twenty-four-hour or timed urine collections are more burdensome. Comper WD et al. Detection of urinary albumin. Adv Chronic Kidney Dis 2005;12:170. [PubMed: 15822052] Miller WG et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem 2009;55:24. [PubMed: 19028824] Ritz E et al. Renal protection in diabetes: lessons from ONTARGET. Cardiovasc Diabetol 2010;9:60. [PubMed: 20920303] |
Aldosterone, Serum
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Aldosterone, serum Salt-loaded (120 meq Na+/d for 3–4 days): Supine: 3–10 ng/dL Upright: 5–30 ng/dL Salt-depleted (10 meq Na+/d for 3–4 days): Supine: 12–36 ng/dL Upright: 17–137 ng/dL SST, Red <SPAN role=presentation tabIndex=0 id=MathJax-Element-4-Frame class=MathJax style="POSITION: relative" data-mathml='‘> Early AM fasting specimen. Separate immediately and freeze. | Aldosterone is the major mineralocorticoid hormone and is a major regulator of extracellular volume and serum potassium concentration. For evaluation of hypoaldosteronism (associated with hyperkalemia), patients should be salt-depleted and upright when specimen is drawn. | Increased in: Primary hyperaldosteronism (2/3 from adrenal hyperplasia, 1/3 from adrenal adenomas) may account for 5–10% of hypertension. Aldosterone/PRA ratio >15 (mL/dL/h) (sensitivity 73–87%, specificity 74–75%) Decreased in: Primary or secondary hypoaldosteronism. | Screening for hyperaldosteronism should use simultaneous determination of serum aldosterone and plasma renin activity (PRA) (see Figure 9–12). In primary aldosteronism, plasma aldosterone is usually elevated whereas PRA is low; in secondary hyperaldosteronism, both serum aldosterone and PRA are usually elevated. The aldosterone/PRA ratio is often used for diagnosis of hyperaldosteronism, but the cutoff value has not been well established and the specificity is low. Mulatero P et al. Evaluation of primary aldosteronism. Curr Opin Endocrinol Diabetes Obes 2010;17:188. [PubMed: 20389241] Mulatero P et al. Confirmatory tests in the diagnosis of primary aldosteronism. Horm Metab Res 2010;42:406. [PubMed: 20119882] Tomaschitz A. Aldosterone to renin ratio—a reliable screening tool for primary aldosteronism? Horm Metab Res 2010;42:382. [PubMed: 20225167] |
Aldosterone, Urine
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Aldosterone, urine* Salt-loaded (120 meq Na+/d for 3–4 days): 1.5–12.5 mcg/24 hr Salt-depleted (20 meq Na+/d for 3–4 days): 18–85 mcg/24 hr [1 mcg/24 hr = 2.77 nmol/d] Bottle containing boric acid <SPAN role=presentation tabIndex=0 id=MathJax-Element-5-Frame class=MathJax style="POSITION: relative" data-mathml='‘> | Secretion of aldosterone is controlled by the renin-angiotensin system. Renin (synthesized and stored in juxtaglomerular cells of kidney) is released in response to both decreased perfusion pressure at the juxtaglomerular apparatus and negative sodium balance. Renin then hydrolyzes angiotensinogen to angiotensin I, which is converted to angiotensin II, which then stimulates the adrenal gland to produce aldosterone. | Increased in: Primary and secondary hyperaldosteronism, some patients with essential hypertension. Decreased in: Primary hypoaldosteronism (eg, 18-hydroxylase deficiency), secondary hypoaldosteronism (hyporeninemic hypoaldosteronism). | Urinary aldosterone is the most sensitive test for primary hyperaldosteronism. Levels >14 mcg/24 hours after 3 days of salt-loading have a 96% sensitivity and 93% specificity for primary hyperaldosteronism: 7% of patients with essential hypertension have urinary aldosterone levels >14 mcg/24 hr after salt-loading. Giacchetti G et al. Analysis of screening and confirmatory tests in the diagnosis of primary aldosteronism: need for a standardized protocol. J Hypertens 2006;24:737. [PubMed: 16531803] Rossi GP et al. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol Metab 2008;19:88. [PubMed: 18314347] Tomaschitz A et al. Aldosterone and arterial hypertension. Nat Rev Endocrinol 2010;6:83. [PubMed: 20027193] |
Alkaline Phosphatase
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Alkaline phosphatase, serum or plasma (ALP) 41–133 IU/L [0.7–2.2 mckat/L] (method- and age-dependent) SST, PPT, green $ | Alkaline phosphatases are primarily found in liver, bone, intestines, kidney, and placenta. Test is used to detect liver disease and bone disorders. | Increased in: Obstructive hepatobiliary disease, bone disease (physiologic bone growth, Paget disease, osteomalacia, osteogenic sarcoma, bone metastases), hyperparathyroidism, rickets, benign familial hyperphosphatasemia, pregnancy (third trimester), GI disease (perforated ulcer or bowel infarct), hepatotoxic drugs. Decreased in: Hypophosphatasia. | Alkaline phosphatase performs well in measuring the extent of bone metastases in prostate cancer. Normal in osteoporosis. Alkaline phosphatase isoenzyme separation by electrophoresis or differential heat inactivation is unreliable. Use γ-glutamyl transpeptidase, which increases in hepatobiliary disease but not in bone disease, to infer origin of increased alkaline phosphatase (ie, liver rather than bone). Aragon G et al. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med 2010;77:195. [PubMed: 20200170] Rajarubendra N et al. Diagnosis of bone metastasis in urological malignancies—an update. Urology 2010;76:782. [PubMed: 20346492] Whyte MP. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann NY Acad Sci 2010;1192:190. [PubMed: 20392236] |
Amebic Serology
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Amebiasis, antibody, serum Negative SST $$ | Test for infection with Entamoeba histolytica (amebiasis) by detection of IgG antibodies that develop 2–4 weeks after infection. Tissue invasion by the organism may be necessary for antibody production. | Increased in: Current or past infection with E. histolytica. Amebic abscess (91%), amebic dysentery (84%), asymptomatic cyst carriers (9%), patients with other diseases, and healthy people (2%). | Seroconversion between acute and convalescent sera is considered evidence of recent infection. A positive antibody test can indicate infection even though stool findings are negative. E. dispar and E. moshkovskii are morphologically indistinguishable from E. histolytica. Only E. histolytica causes disease in humans. Molecular tests are now available to distinguish between them for research and epidemiologic purposes. Ali K et al. Molecular epidemiology of amebiasis. Infect Genet Evol 2008;8:698. [PubMed: 18571478] van Doorn HR et al. Use of rapid dipstick and latex agglutination tests and enzyme-linked immunosorbent assay for serodiagnosis of amebic liver abscess, amebic colitis, and Entamoeba histolytica cyst passage. J Clin Microbiol 2005;43:4801. [PubMed: 16145144] |
Ammonia
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Ammonia, plasma (NH3) 18–60 mcg/dL [11–35 mcmol/L] Green $$ Separate plasma from cells immediately. Avoid hemolysis. Analyze immediately. Place on ice. | Ammonia is liberated by bacteria in the large intestine or by protein metabolism and is rapidly converted to urea in the liver. In liver disease or portal-systemic shunting, the blood ammonia concentration increases. In acute liver failure, elevation of blood ammonia may cause brain edema; in chronic liver failure, it may be responsible for hepatic encephalopathy. | Increased in: Liver failure, hepatic encephalopathy (especially if protein consumption is high or if there is GI bleeding), fulminant hepatic failure, Reye syndrome, portacaval shunting, cirrhosis, urea cycle metabolic defects, urea-splitting urinary tract infection with urinary diversion, and organic acidemias. Drugs: diuretics, acetazolamide, asparaginase, fluorouracil (transient), others. Spuriously increased by any ammonia-containing detergent on laboratory glassware. Decreased in: Decreased production by gut bacteria (kanamycin, neomycin). Decreased gut absorption (lactulose). | Plasma ammonia level correlates poorly with degree of hepatic encephalopathy in chronic liver disease. Test not useful in adults with known liver disease. Ammonia toxicity is probably mediated by glutamine, synthesized in excess from ammonia and glutamate. Albrecht J et al. Glutamine as a mediator of ammonia neurotoxicity: a critical appraisal. Biochem Pharmacol 2010;80:1303. [PubMed: 20654582] Prakash R et al. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol 2010;7:515. [PubMed: 20703237] Wilkinson DJ et al. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog Neurobiol 2010;91:200. [PubMed: 20138956] |
Amylase
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Amylase, serum or plasma 20–110 U/L [0.33–1.83 mckat/L] (laboratory-specific) SST, PPT $ | Amylase hydrolyzes complex carbohydrates. Serum amylase is derived primarily from pancreas and salivary glands and is increased with inflammation or obstruction of these glands. Other tissues have some amylase activity, including ovaries, small and large intestine, and skeletal muscle. | Increased in: Acute pancreatitis (70–95%), pancreatic pseudocyst, pancreatic duct obstruction (cholecystitis, choledocholithiasis, pancreatic carcinoma, stone, stricture, duct sphincter spasm), bowel obstruction and infarction, mumps, parotitis, diabetic ketoacidosis, penetrating peptic ulcer, peritonitis, ruptured ectopic pregnancy, macroamylasemia. Drugs: azathioprine, hydrochlorothiazide. Decreased in: Pancreatic insufficiency, cystic fibrosis. Usually normal or low in chronic pancreatitis. | Macroamylasemia is indicated by high serum but low urine amylase. Serum or plasma lipase is an alternative test for acute pancreatitis. It has clinical sensitivity equivalent to that of amylase but with better specificity. There is no advantage to performing both tests. Amylase isoenzymes are not of practical use because of technical problems. Carroll JK et al. Acute pancreatitis: diagnosis, prognosis, and treatment. Am Fam Physician 2007;75:1513. [PubMed: 17555143] Matull WR et al. Biochemical markers of acute pancreatitis. J Clin Pathol 2006;59:340. [PubMed: 16567468] Shah AM et al. Acute pancreatitis with normal serum lipase: a case series. JOP 2010;11(4):369. [PubMed: 20601812] |
Angiotensin-Converting Enzyme
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Angiotensin-converting enzyme, serum (ACE) 200–590 nkat/L (method-dependent) SST, red $$ | ACE is a dipeptidyl carboxypeptidase that converts angiotensin I to the vasopressor, angiotensin II. ACE is normally present in the kidneys and other peripheral tissues. Serum levels in healthy subjects are dependent on polymorphisms in ACE genes. In granulomatous disease, ACE levels increase, derived from epithelioid cells within granulomas. | Increased in: Sarcoidosis, hyperthyroidism, acute hepatitis, primary biliary cirrhosis, diabetes mellitus, multiple myeloma, osteoarthritis, amyloidosis, Gaucher disease, pneumoconiosis, histoplasmosis, miliary tuberculosis. Drugs: dexamethasone. Decreased in: Renal disease, obstructive pulmonary disease, hypothyroidism. | Test is not useful as a screening test for sarcoidosis (low sensitivity). Specificity is compromised by positive tests in diseases more common than sarcoidosis. Some advocate measurement of ACE to follow disease activity in sarcoidosis. Biller H et al. Gene polymorphisms of ACE and the angiotensin receptor AT2R1 influence serum ACE levels in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2009;26:139. [PubMed: 20560294] Herbort CP et al; members of Scientific Committee of First International Workshop on Ocular Sarcoidosis. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop on Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm 2009;17:160. [PubMed: 19585358] |
Antibody Screen
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Antibody screen, serum or plasma Red or lavender/pink $ Properly identified and labeled blood specimens are critical. | Detects antibodies to non-ABO red blood cell antigens in recipient’s serum or plasma, using reagent red cells selected to possess antigens against which common antibodies can be produced. Further identification of the specificity of any antibody detected (using panels of red cells of known antigenicity) makes it possible to test donor blood for the absence of the corresponding antigen. Primary response to first antigen exposure requires 20–120 days; antibody is largely IgM with a small quantity of IgG. Secondary response requires 1–14 days; antibody is mostly IgG. | Positive in: Presence of alloantibody, autoantibodies. | In practice, a type and screen (ABO and Rh grouping and antibody screen) is adequate work-up for patients undergoing operative procedures unlikely to require transfusion. A negative antibody screen implies that a recipient can receive type-specific (ABO-Rh identical) blood with minimal risk. Some antibody activity (eg, anti-Jka, anti-E) may become so weak as to be undetectable but increase rapidly after secondary stimulation with the same antigen. Technical Manual of the American Association of Blood Banks, 17th ed. American Association of Blood Banks, 2011. |
Antidiuretic Hormone
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Antidiuretic hormone, plasma (ADH) If serum osmolality > 290 mosm/kg H2O: 2–12 pg/mL If serum osmolality <290 mosm/kg H2O: <2 pg/mL Lavender, pink <SPAN role=presentation tabIndex=0 id=MathJax-Element-6-Frame class=MathJax style="POSITION: relative" data-mathml='‘> Draw in two chilled tubes and deliver to lab on ice. Specimen for serum osmolality must be drawn at same time. | Antidiuretic hormone, also known as arginine vasopressin hormone, is a hormone secreted from the posterior pituitary that acts on the distal nephron to conserve water and regulate the tonicity of body fluids. Water deprivation provides both an osmotic and a volume stimulus for ADH release by increasing plasma osmolality and decreasing plasma volume. Water administration lowers plasma osmolality and expands blood volume, inhibiting the release of ADH by the osmoreceptor and the atrial volume receptor mechanisms. Copeptin, the C-terminal part of the AVP precursor peptide, is more stable and may serve a sensitive surrogate marker for ADH release. | Increased in: Nephrogenic diabetes insipidus, syndrome of inappropriate antidiuretic hormone (SIADH). Drugs: nicotine, morphine, chlorpropamide, clofibrate, cyclophosphamide. Normal relative to plasma osmolality in: Primary polydipsia. Decreased in: Central (neurogenic) diabetes insipidus. Drugs: ethanol, phenytoin. | Test very rarely indicated. Measurement of serum and urine osmolality usually suffices. Test not indicated in diagnosis of SIADH. Patients with SIADH show decreased plasma sodium and decreased plasma osmolality, usually with high urine osmolality relative to plasma. These findings in a normovolemic patient with normal thyroid and adrenal function are sufficient to make the diagnosis of SIADH without measuring ADH itself. Fenske W et al. The syndrome of inappropriate secretion of antidiuretic hormone: diagnostic and therapeutic advances. Horm Metab Res 2010;42:691. [PubMed: 20607641] Levtchenko EN et al. Nephrogenic syndrome of inappropriate antidiuresis. Nephrol Dial Transplant 2010;25:2839. [PubMed: 20543212] |
Antiglobulin Test, Direct
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Antiglobulin test, direct, red cells (direct Coombs, DAT) Negative Lavender/pink or red $ Blood anticoagulated with EDTA is used to prevent in vitro uptake of complement components. A red top tube may be used, if necessary. | Direct antiglobulin test is used to demonstrate in vivo coating of red cells with globulins, in particular IgG and C3d. DAT is performed with a polyspecific reagent that detects both IgG and C3d. If positive, tests with monospecific reagents (anti-IgG and anti-complement) should be performed to characterize the immune process involved. | Positive in: Autoimmune hemolytic anemia, hemolytic disease of the newborn, alloimmune reactions to recently transfused cells, and drug-induced hemolysis. Drugs may induce the formation of antibodies, either against the drug itself or against intrinsic red cell antigens. This may lead to a positive DAT, immune red cell destruction, or both. Some of the antibodies produced appear to be dependent on the presence of the drug (eg, penicillin, quinidine, ceftriaxone), whereas others are independent of the continued presence of the inciting drug (eg, methyldopa, levodopa, procainamide, cephalosporins, fludarabine). | A positive DAT implies in vivo red cell coating by immunoglobulins or complement. Such red cell coating may or may not be associated with immune hemolytic anemia. The DAT can detect a level of 100–500 molecules of IgG per red cell and 400–1100 molecules of C3d per red cell, depending on the reagent and technique used. Positive DATs without clinical manifestations of immune-mediated red cell destruction are reported in the range of 1 in 1000 up to 1 in 14,000 blood donors and 1–15% of hospital patients. A false-positive DAT is often seen in patients with hypergammaglobulinemia, eg, in some HIV-positive patients. Technical Manual of the American Association of Blood Banks, 17th ed. American Association of Blood Banks, 2011. |
Antiglobulin Test, Indirect
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Antiglobulin test, indirect, serum or plasma (indirect Coombs) Negative Red or lavender, pink $ | Indirect antiglobulin test is used to demonstrate the presence in the patient’s serum/plasma of unexpected antibody to ABO and Rh-compatible reagent red blood cells. Patient serum or plasma is incubated in vitro with reagent red cells, which are then washed to remove unbound globulins. Agglutination that occurs when antihuman globulin (AHG, Coombs) reagent is added indicates that antibody has bound to a specific antigen present on the red cells. | Positive in: Presence of alloantibody or autoantibody. Drugs: methyldopa. | The technique is used in antibody detection and identification, and in the AHG crossmatch prior to transfusion (see Type and crossmatch). Technical Manual of the American Association of Blood Banks, 17th ed. American Association of Blood Banks, 2011. |
Antistreptolysin O
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Antistreptolysin O, serum (ASO) 0–1 year: <200 IU/mL; 2–12 years: <240 IU/mL 13 years or older: <330 IU/mL (laboratory-specific) SST $$ | Detects the presence of antibody to the antigen streptolysin O produced by group A streptococci. Streptococcal antibodies appear about 2 weeks after infection. Titer rises to a peak at 4–6 weeks and may remain elevated for 6 months to 1 year. Test is based on the neutralization of hemolytic activity of streptolysin O toxin by antistreptolysin O antibodies in serum. | Increased in: Recent infection with group A β-hemolytic streptococci: scarlet fever, erysipelas, streptococcal pharyngitis/tonsillitis (40–50%), rheumatic fever (80–85%), poststreptococcal glomerulonephritis. Some collagen vascular diseases. Certain serum lipoproteins, bacterial growth products, or oxidized streptolysin O may result in inhibition of hemolysis and thus cause false-positive results. | Standardization of (Todd) units may vary significantly from laboratory to laboratory. ASO titers are not useful in management of acute streptococcal pharyngitis. In patients with rheumatic fever, test may be a more reliable indicator of recent streptococcal infection than throat culture. An increasing titer is more suggestive of acute streptococcal infection than a single elevated level. Even with severe infection, ASO titers rise in only 70–80% of patients. ASO and anti-DNase-B together increase test sensitivity. Normal range increases with age. Hahn RG et al. Evaluation of poststreptococcal illness. Am Fam Physician 2005;71:1949. [PubMed: 15926411] Jeng A et al. The role of beta hemolytic streptococci in causing diffuse non-culturable cellulitis: a prospective investigation. Medicine (Baltimore) 2010;89:217. [PubMed: 20616661] |
Antithrombin
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Antithrombin (AT), plasma 84–123% (enzymatic activity, qualitative) 80–130% (antigen, quantitative) Blue $$ Transport to lab on ice. Plasma must be separated and frozen in a polypropylene tube within 2 hours. | Antithrombin is a serine protease inhibitor that protects against thrombus formation by inhibiting thrombin and other factors, including IXa, Xa, XIa. It accounts for 70–90% of the anticoagulant activity of human plasma. Its activity is enhanced 1000-fold by heparin. There are two types of assay: functional/enzymatic (activity) and immunologic (antigen). Since the immunologic assay cannot rule out functional AT deficiency, a functional assay should be ordered first. Functional assays test AT activity in inhibiting thrombin or factor Xa. Given an abnormal functional assay, the immunologic test indicates whether there is decreased production of AT (type I deficiency) or intact synthesis of a dysfunctional protein (type II deficiency). | Decreased in: Congenital and acquired AT deficiency (nephrotic syndrome, chronic liver disease), oral contraceptive use, chronic disseminated intravascular coagulation (DIC), acute venous thrombosis (consumption), L-asparginase treatment (decreased synthesis) and heparin therapy (consumption). | Congenital or acquired AT deficiency results in a hypercoagulable state, venous thromboembolism, and heparin resistance. Congenital AT deficiency is present in 1:2000–1:3000 people and is autosomal codominant. Heterozygotes have AT levels 20–60% of normal. Evaluation of AT should be considered in patients with venous thrombosis, especially for thrombosis in unusual sites or associated with heparin resistance. Testing should be performed at least 2 months after the thrombotic event, at a time when the patient is not receiving anticoagulants. De Stefano V et al. The risk of recurrent venous thromboembolism in patients with inherited deficiency of natural anticoagulants antithrombin, protein C and protein S. Haematologica 2006;91:695. [PubMed: 16670075] Khor B et al. Laboratory tests for antithrombin deficiency. Am J Hematol 2010;85:947. [PubMed: 21108326] Rodgers GM. Role of antithrombin concentrate in treatment of hereditary antithrombin deficiency. An update. Thromb Haemost 2009;101:806. [PubMed: 19404531] |
α1-Antitrypsin
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
α1-Antitrypsin (α1-Antiprotease) serum or plasma 110–270 mg/dL [1.1–2.7 g/L] SST, red, PPT, lavender, pink $$ | α1-Antitrypsin is an α1 globulin glycoprotein serine protease inhibitor (Pi) whose deficiency leads to excessive protease activity and panacinar emphysema in adults or liver disease in children (seen as ZZ and SZ phenotypes). Cirrhosis of the liver and liver cancer in adults are also associated with the Pi Z phenotype. | Increased in: Inflammation, infection, rheumatic disease, malignancy, and pregnancy as an acute-phase reactant. Decreased in: Congenital α1-antitrypsin deficiency, nephrotic syndrome. | Smoking is a much more common cause of chronic obstructive pulmonary disease in adults than is α1-antitrypsin deficiency. Testing for α1-antitrypsin deficiency should be done in young patients (<50 year-old with exercise limitation from emphysema), those with emphysema in absence of cigarette smoking, and in presence of familial clustering or basilar predominance of emphysema. Bals R. Alpha-1-antitrypsin deficiency. Best Pract Res Clin Gastroenterol 2010;24:629. [PubMed: 20955965] Ferrarotti I et al. Laboratory diagnosis of alpha-1-antitrypsin deficiency. Transl Res 2007;150:267. [PubMed: 17964515] Fromer L. Improving diagnosis and management of alpha-1- antitrypsin deficiency in primary care: translating knowledge into action. COPD 2010;7(3):192. [PubMed: 20486818] Kelly E et al. Alpha-1-antitrypsin deficiency. Respir Med 2010 104(6):763. [PubMed: 20303723] Miravitlles M et al. Laboratory testing of individuals with severe alpha-1-antitrypsin deficiency in three European centres. Eur Respir J 2010;35(5):960. [PubMed: 20436173] Pietrangelo A. Inherited metabolic disease of the liver. Curr Opin Gastroenterol 2009;25(3):2094. [PubMed: 19342951] Sandhaus RA. Alpha-1-antitrypsin deficiency: whom to test, whom to treat? Semin Respir Crit Care Med 2010;31:343. [PubMed: 20496303] |
Arterial Blood Gases
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Arterial blood gases (ABG), whole blood Heparinized syringe $$$ Collect arterial blood in a heparinized syringe, and send to laboratory immediately. | Blood gas measurements provide information about cardiopulmonary (oxygen and carbon dioxide exchange) and metabolic (acid-base) status. When integrated with the history and physical examination, the rapidly available arterial blood gas (ABG) analysis is useful in the resuscitation of the acutely ill or injured patient. | See Carbon Dioxide, Oxygen, and pH. | Panos RJ et al. Exertional desaturation in patients with chronic obstructive pulmonary disease. COPD 2009;6:478. [PubMed: 19938972] |
Aspartate Aminotransferase
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Aspartate aminotransferase, serum or plasma (AST, SGOT, GOT) 0–35 IU/L [0–0.58 mckat/L] (laboratory-specific) SST, PPT, green, lavender $ | Intracellular enzyme involved in amino acid metabolism. Present in large concentrations in liver, skeletal muscle, brain, red cells, and heart. Released into the bloodstream when tissue is damaged, especially in liver injury. | Increased in: Acute viral hepatitis (ALT > AST), biliary tract obstruction (cholangitis, choledocholithiasis), alcoholic hepatitis and cirrhosis (AST > ALT), liver abscess, metastatic or primary liver cancer; right heart failure, ischemic or hypoxic injury to liver (“shock liver”), extensive trauma. Drugs that cause cholestasis or hepatotoxicity. Decreased in: Pyridoxine (vitamin B6) deficiency. | Test is not indicated for diagnosis of myocardial infarction. AST/ALT ratio >1 suggests cirrhosis in patients with hepatitis C. See Liver function tests (Table 8–14). Giannini EG et al. Liver enzyme alteration: a guide for clinicians. CMAJ 2005;172:367. [PubMed: 15684121] Ozer J et al. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008;245:194. [PubMed: 18291570] Senior JR. Monitoring for hepatotoxicity: what is the predictive value of liver “function” tests? Clin Pharmacol Ther 2009;85:331. [PubMed: 19129750] |
B Cell Immunoglobulin Heavy-Chain (IgH) Gene Rearrangement
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
B-cell immunoglobulin heavy-chain (IgH) gene rearrangement Whole blood, bone marrow, frozen or paraffin-embedded tissue Lavender <SPAN role=presentation tabIndex=0 id=MathJax-Element-7-Frame class=MathJax style="POSITION: relative" data-mathml='‘> | In general, the percentage of B lymphocytes with identical immunoglobulin heavy-chain gene rearrangements is very low; in malignancies, however, the clonal expansion of one population leads to a large number of cells with identical B-cell immunoglobulin heavy-chain gene rearrangements. B-cell clonality can be assessed by restriction fragment Southern blot hybridization or more commonly polymerase chain reaction (PCR). | Positive in: B-cell neoplasms such as lymphoma (monoclonal B-cell proliferation), plasma cell neoplasms. | The diagnostic sensitivity and specificity are heterogeneous and laboratory- and method-specific. Results of the test must always be interpreted in the context of morphologic and other relevant data (eg, flow cytometry) and should not be used alone for a diagnosis of malignancy. The test is primarily for initial diagnosis, but may also be used to detect minimal residual disease. Bagg A et al. Immunoglobulin heavy chain gene analysis in lymphomas: a multi-center study demonstrating the heterogeneity of performance of polymerase chain reaction assays. J Mol Diagn 2002;4:81. [PubMed: 11986398] Garcia-Castillo H et al. Detection of clonal immunoglobulin and T-cell receptor gene recombination in hematological malignancies: monitoring minimal residual disease. Cardiovasc Hematol Disord Drug Targets 2009;9:124. [PubMed: 19519371] |
BCR-ABL, t(9;22) Translocation by RT-PCR
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
BCR-ABL, t(9;22) translocation by RT-PCR, qualitative Blood Lavender <SPAN role=presentation tabIndex=0 id=MathJax-Element-8-Frame class=MathJax style="POSITION: relative" data-mathml='‘> | Approximately 95% of cases of chronic myelogenous leukemia (CML) have the characteristic t(9;22)(q34;q11) that results in a BCR-ABL gene fusion on the derived chromosome 22 called the Philadelphia (Ph) chromosome. The remaining cases either have a cryptic translocation between 9q34 and 22q11 that cannot be identified by routine cytogenetic analysis, or have variant translocations involving a third or even a fourth chromosome besides 9 and 22. The BCR-ABL fusion transcript is found in all cases of CML, including those with a cryptic or variant translocation. A subset of acute lymphoblastic leukemia (ALL) and occasionally acute myelogenous leukemia (AML, mostly CML blast crisis) also have the Ph chromosome, and therefore are positive for BCR-ABL, t(9;22) translocation. | Positive in: All CML, a subset of acute lymphoblastic leukemia (ALL), and rare acute myeloid leukemia (eg, CML blast crisis). | This assay can also be used to distinguish between the major and minor transcripts. The major transcript, characterized by the p210 fusion gene product, is typically detected in CML. The minor transcript, characterized by the p190 fusion gene product, is typically detected in ALL. Detection limit of RT-PCR based assays is at least 1 in 100,000 cells. Small amounts of p190 transcript can be detected in most patients with CML, due to alternative splicing of the BCR gene. For treatment monitoring, the BCR-ABL, t(9;22) translocation quantitative RT-PCR assay should be used. The quantitative assay may not distinguish between the major and minor BCR-ABL products. Foroni L et al. Technical aspects and clinical applications of measuring BCR-ABL1 transcripts number in chronic myeloid leukemia. Am J Hematol 2009;84:517. [PubMed: 19544476] Goldman JM et al. BCR-ABL in chronic myelogenous leukemia–how does it work? Acta Haematol 2008;119:212. [PubMed: 18566539] Ross DM et al. Current and emerging tests for the laboratory monitoring of chronic myeloid leukaemia and related disorders. Pathology 2008;40:231. [PubMed: 18428043] |
BCR-ABL Mutation Analysis
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
BCR-ABL mutation analysis (BCR-ABL genotyping) Blood Lavender <SPAN role=presentation tabIndex=0 id=MathJax-Element-9-Frame class=MathJax style="POSITION: relative" data-mathml='‘> | The analysis involves direct DNA sequencing of the PCR-amplified BCR-ABL products. The sequence is then compared with an ABL kinase domain reference sequence to identify single or multiple mutations. | Positive in: Imatinib-resistant chronic myeloid leukemia; imatinib-resistant Ph-positive precursor B-lymphoblastic leukemia. | The BCR-ABL tyrosine kinase inhibitor imatinib is generally effective in Philadelphia chromosome–positive (Ph-positive) leukemias (eg, chronic myeloid leukemia, CML). However, patients may have an inferior response to imatinib, either failing to respond to primary therapy or demonstrating progression (or relapse) after an initial response. Imatinib resistance is mainly due to leukemic subclones with BCR-ABL mutation(s) in the ABL kinase domain that interfere with imatinib binding. The BCR-ABL mutation analysis can assist physicians in evaluating resistance to imatinib therapy and facilitate appropriate adjustments to treatment (eg, increase in imatinib dosage or switch to other tyrosine kinase inhibitors). Mutations at 17 different amino acid positions within the BCR-ABL kinase domain have been associated with clinical resistance to imatinib. Patients with T315I mutation are also resistant to dasatinib and nilotinib. Bixby D et al. Seeking the causes and solutions to imatinib-resistance in chronic myeloid leukemia. Leukemia 2011;25:7. [PubMed: 21102425] Hughes TP et al. Monitoring disease response to tyrosine kinase inhibitor therapy in CML. Hematology Am Soc Hematol Educ Program 2009:477. [PubMed: 20008233] |
Beta-hCG
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Beta-hCG, quantitative, serum Males and nonpregnant females: undetectable or <5 mIU/mL [IU/L] SST, red $$ | Human chorionic gonadotropin (hCG) is a glycoprotein made up of two subunits (α and β). The β-subunit is specific for hCG. hCG is produced by trophoblastic tissue, and its detection in serum or urine is the basis for pregnancy testing. Serum hCG can be detected as early as 24 hours after implantation at a concentration of 5 mIU/mL. During normal pregnancy, serum levels double every 2–3 days and are 50–100 mIU/mL at the time of the first missed menstrual period. Peak levels are reached 60–80 days after the last menstrual period (LMP) (30,000–100,000 mIU/mL), and levels then decrease to a plateau of 5,000–10,000 mIU/mL at about 120 days after LMP and persist until delivery. Regular hCG produced by differentiated syncytotrophoblast cells primarily functions to promote progesterone production and to maintain the myometrial and the vascular supply of the placenta during the first trimester. Hyperglycosylated hCG (hCG-H) is produced by undifferentiated extravillous cytotrophoblast cells and maintains trophoblast invasion as in implantation of pregnancy. Hyperglycosylated hCG and/or free β-subunit are produced by a high proportion of malignant gestational trophoblastic diseases. | Increased in: Pregnancy (including ectopic pregnancy), hyperemesis gravidarum, trophoblastic tumors (hydatidiform mole, choriocarcinoma), some germ cell tumors (teratomas, seminoma), ectopic hCG production by other malignancies. Decreasing over time: Threatened abortion. | Routine pregnancy testing is done by qualitative urine hCG test, or less commonly quantitative serum hCG test. Test is positive (>50 mIU/mL) in most pregnant women at the time of or shortly after the first missed menstrual period. Quantitative hCG test detects hCG levels as low as 1.0 mIU/mL. It is preferred for the evaluation of suspected ectopic pregnancy and threatened abortion. In both situations, hCG levels fail to demonstrate the normal early pregnancy increase. Test is also indicated for following the course of trophoblastic and germ cell tumors. Most commercially available hCG tests detect only regular hCG. In patients with malignancies that produce primarily hCG-H, the test should be interpreted with caution. Chung K et al. The use of serial human chorionic gonadotropin levels to establish a viable or a nonviable pregnancy. Semin Reprod Med 2008;26:383. [PubMed: 18825606] Cole LA. Human chorionic gonadotropin tests. Expert Rev Mol Diagn 2009;9:721. [PubMed: 19817556] Cole LA. Hyperglycosylated hCG, a review. Placenta 2010;31:653. [PubMed: 20619452] Nama V et al. Tubal ectopic pregnancy: diagnosis and management. Arch Gynecol Obstet 2009;279:443. [PubMed: 18665380] |
Bilirubin
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Bilirubin, serum or plasma 0.1–1.2 mg/dL [2–21 mcmol/L] Direct (conjugated to glucuronide) bilirubin: 0.1–0.4 mg/dL [<7 mcmol/L]; Indirect (unconjugated) bilirubin: 0.2–0.7 mg/dL [<12 mcmol/L] SST, PPT $$ | Bilirubin is the orange-yellow pigment derived from the breakdown of hemoglobin (heme). The majority of bilirubin comes from senescent red cells. It is biotransformed in the liver and excreted in bile and urine. The conjugated form is water-soluble and reacts directly with diazo dyes in the absence of reaction accelerator, and is therefore called direct bilirubin. The unconjugated form is fat-soluble and reacts with diazo dyes only in the presence of accelerator; so it is called indirect. Some conjugated bilirubin is bound to serum albumin, so-called D (delta) bilirubin. | Increased in: Acute or chronic hepatitis, cirrhosis, biliary tract obstruction, toxic hepatitis, neonatal jaundice (neonatal hyperbilirubinemia), congenital liver enzyme abnormalities (Dubin-Johnson, Rotor, Gilbert, Crigler-Najjar syndromes), fasting, hemolytic disorders. Hepatotoxic drugs. | Assay of total bilirubin includes conjugated (direct) and unconjugated (indirect) bilirubin. The unconjugated (indirect) form is the difference between total bilirubin (with reaction accelerator) and the direct bilirubin fraction. Delta bilirubin is determined together with conjugated bilirubin. Delta bilirubin (half-life is about 17 days) accounts for relatively slow regression of jaundice. Only conjugated bilirubin appears in the urine, and it is indicative of liver disease and biliary tract obstruction. Hemolysis is associated with increased unconjugated bilirubin. Unbound (free) serum or plasma bilirubin level correlates better than total bilirubin with CNS bilirubin concentrations and bilirubin encephalopathy (kernicterus) in newborn jaundice. Ahlfors CE et al. Unbound (free) bilirubin: improving the paradigm for evaluating neonatal jaundice. Clin Chem 2009;55:1288. [PubMed: 19423734] Cohen RS et al. Understanding neonatal jaundice: a perspective on causation. Pediatr Neonatol 2010;51:143. [PubMed: 20675237] Fevery J. Bilirubin in clinical practice: a review. Liver Int 2008; 28:592. [PubMed: 18433389] |
Blood Urea Nitrogen
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Blood urea nitrogen, serum or plasma (BUN) 8–20 mg/dL [2.9–7.1 mmol/L] SST, PPT, green $ | Urea is the end product of protein metabolism, which is excreted by the kidney. BUN is directly related to protein intake and nitrogen metabolism and inversely related to the rate of excretion of urea. Urea concentration in glomerular filtrate is the same as in plasma, but its tubular reabsorption is inversely related to the rate of urine formation. Thus, BUN is a less useful measure of glomerular filtration rate than the serum/plasma creatinine (Cr). | Increased in: Renal failure (acute or chronic), urinary tract obstruction, dehydration, shock, burns, CHF, GI bleeding, nephrotoxic drugs (eg, gentamicin). Decreased in: Hepatic failure, nephrotic syndrome, cachexia (low-protein and high-carbohydrate diets). | Urease assay method is commonly used. Blood BUN/Cr ratio (normally 10:1–20:1) is decreased in acute tubular necrosis, advanced liver disease, low protein intake, and following hemodialysis. Blood BUN/Cr ratio is increased in dehydration, GI bleeding, and increased catabolism. Edelstein CL. Biomarkers of acute kidney injury. Adv Chronic Kidney Dis 2008;15:222. [PubMed: 18565474] Waikar SS et al. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 2008;3:844. [PubMed: 18337550] |
B-Type Natriuretic Peptide
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
B-type natriuretic peptide (BNP), plasma Lavender, pink 0–100 pg/mL [0–347 pmol/L] $$ Point-of-care immunoassays also available. | BNP has biologic effects similar to those of atrial natriuretic peptide (ANP) and is stored mainly in the myocardium of the cardiac ventricles. Blood BNP levels are elevated in hypervolemic states such as congestive heart failure (CHF). BNP is useful for guiding and monitoring heart failure treatment and for predicting prognosis. Clinical applications in the setting of CHF include: to determine the cause of symptoms (eg, dyspnea); to estimate the degree of severity of heart failure; to estimate the risk of disease progression; and to screen for less symptomatic disease in high-risk populations. | Increased in: CHF (cutoff concentration: >100 pg/mL yields a sensitivity of 90%, specificity, 73%. BNP <100 pg/mL has a negative predictive value of 90%. BNP >400 pg/mL suggests CHF with specificity exceeding 90%). BNP is also increased in a variety of other cardiac and noncardiac diseases including acute coronary syndrome, left ventricular dysfunction, valvular aortic stenosis, pulmonary embolism, and renal insufficiency. | BNP testing is not a substitute for careful cardiopulmonary evaluation and should not be the sole criterion for admission/discharge of a patient. Although normal levels indicate a low probability of CHF, they do not exclude it or other serious cardiopulmonary disorders. Moderately increased levels are not specific for CHF and can occur with a variety of cardiac and noncardiac diseases. BNP is not recommended for screening for left ventricular dysfunction or hypertrophy in the general population. It is also unnecessary to test BNP in patients with obvious CHF (eg, NYHA class IV). Treatment of CHF has been reported to decrease BNP levels in parallel with clinical improvement. Tests for N-terminal fragment of pro-BNP (NT-pro-BNP) are also available, and diagnostic performance is comparable to that of BNP. The normal reference intervals of pro-BNP are laboratory-dependent and vary with age and sex. Maisel A et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 2008;10:824. [PubMed: 18760965] McCullough PA et al. An evidence-based algorithm for the use of B-type natriuretic testing in acute coronary syndromes. Rev Cardiovasc Med 2010;11(Suppl 2):S51. [PubMed: 20700103] Palazzuoli A et al. Natriuretic peptides (BNP and NT-proBNP): measurement and relevance in heart failure. Vasc Health Risk Manag 2010;6:411. [PubMed: 20539843] Porapakkham P et al. B-type natriuretic peptide-guided heart failure therapy: a meta-analysis. Arch Intern Med. 2010;170(6):507. [PubMed: 20308637] |
Brucella Antibody
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Brucella antibodies, serum Negative SST, red $ | Patients with acute brucellosis generally develop an agglutinating antibody titer of >1:160 within 3 weeks. The titer may rise during the acute infection, with relapses, brucellergin skin testing, or use of certain vaccines (see Interpretation). The agglutinin titer usually declines after 3 months or after successful therapy. Low titers may persist for years. Indirect enzyme-linked immunosorbent assay (ELISA) measuring IgM, IgG, and IgA antibodies have higher sensitivity and specificity than the agglutinating antibody test. Routine use of PCR and RT-PCR assays for diagnosis of human brucellosis needs further clinical evaluation. | Positive in: Brucella infection (except B. canis) (97% within 3 weeks of illness); recent brucellergin skin test; infections with Francisella tularensis, Yersinia enterocolitica, salmonella, Rocky Mountain spotted fever; vaccinations for cholera and tularemia. Negative in: B. canis infection. | This test detects antibodies against all of the Brucella species except B. canis. A fourfold or greater rise in titer in separate specimens drawn 1–4 weeks apart is indicative of recent exposure. Since titers can remain high for a prolonged period, they are not suitable for patient follow-up. Specimens testing positive or equivocal for Brucella antibodies by ELISA should be confirmed by bacterial agglutination. Final diagnosis depends on isolation of organism by culture. Araj GF. Update on laboratory diagnosis of human brucellosis. Int J Antimicrob Agents 2010;36(Suppl 1):S12. [PubMed: 20692128] Franco MP et al. Human brucellosis. Lancet Infect Dis 2007;7:775. [PubMed: 18045560] |
C-Peptide
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
C-peptide, serum or plasma 0.8–4.0 ng/mL [mcg/L] (0.26–1.3 nmol/L) SST, PPT, lavender, green $$$ Fasting sample preferred. | C-peptide is an inactive by-product of the cleavage of proinsulin to active insulin. Its presence indicates endogenous release of insulin. The half-life of C-peptide in the blood is about 30 min. C-peptide is largely excreted by the kidney. | Increased in: Renal failure, ingestion of oral hypoglycemic drugs, insulinomas, Beta-cell transplants. Decreased in: Factitious hypoglycemia due to insulin administration, pancreatectomy, type 1 diabetes mellitus (decreased or undetectable). | Test is most useful to detect factitious insulin injection (increased insulin, decreased C-peptide) or endogenous insulin production in diabetic patients receiving insulin (C-peptide present). A random C-peptide level has reasonable discriminatory power for determining type 1 vs type 2 diabetes. A molar ratio of insulin to C-peptide >1.0 in peripheral venous blood in a hypoglycemic patient is consistent with surreptitious or inadvertent insulin administration but not insulinoma. C-peptide levels of 2 nmol/L or greater suggest insulinoma. Cryer PE et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009;94:709. [PubMed: 19088155] Hills CE et al. C-peptide as a therapeutic tool in diabetic nephropathy. Am J Nephrol 2010;31:389. [PubMed: 20357430] Marks V. Murder by insulin: suspected, purported and proven— a review. Drug Test Anal 2009;1:162. [PubMed: 20355194] |
C-Reactive Protein, High Sensitivity
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
C-reactive protein, high sensitivity (hs-CRP), serum or plasma <1.0 mg/dL (lower 95th percentile) SST, PPT, green $ | CRP is an acute-phase reactant protein. Hepatic secretion is stimulated in response to inflammatory cytokines. Unlike other acute-phase proteins, CRP is not affected by hormones. CRP activates the complement system, binds to Fc receptors, and serves as an opsonin for some microorganisms. Rapid, marked increases in CRP occur with inflammation, infection, trauma and tissue necrosis, malignancies, and autoimmune disorders. CRP levels are also valuable in assessing vascular inflammation and cardiovascular risk stratification. CRP level has been shown to be an independent risk factor for atherosclerotic disease. Elevated CRP levels are associated with increased cardiovascular morbidity and mortality in patients with coronary artery disease. | Increased in: Inflammatory states, including arteriosclerotic disorders. | CRP is a very sensitive but nonspecific marker of inflammation. A variety of conditions other than arteriosclerosis may cause dramatic increases in CRP levels. CRP levels increase within 2 hours of acute insult (eg, surgery, infection) and should peak and begin decreasing within 48 hours if no other inflammatory event occurs. In patients with rheumatoid arthritis, persistently elevated CRP concentrations are present when the disease is active and usually fall to normal during periods of complete remission. Patients with high hs-CRP concentrations are more likely to develop stroke, myocardial infarction, and severe peripheral vascular disease. hs-CRP results are used to assign risk as follows: <1.0 mg/L lowest tertile, lowest risk; 1.0-3.0 mg/L middle tertile, average risk; >3.0 mg/L highest tertile, highest risk. Noncardiovascular cause should be considered if CRP values are >10 mg/dL with repeat measurements. Bajpai A et al. Should we measure C-reactive protein on earth or just on JUPITER? Clin Cardiol 2010;33:190. [PubMed: 20394038] Devaraj S et al. Role of C-reactive protein in contributing to increased cardiovascular risk in metabolic syndrome. Curr Atheroscler Rep. 2010;12:110. [PubMed: 20425246] Kaysen GA. Biochemistry and biomarkers of inflamed patients: why look, what to assess. Clin J Am Soc Nephrol 2009;4(Suppl 1):S56. [PubMed: 19996007] |
C1 Esterase Inhibitor
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
C1 esterase inhibitor (C1 INH), serum 20-40 mg/dL (method-dependent) SST $$ | C1 esterase inhibitor (C1 INH) is a broad-spectrum protease inhibitor, which controls the first stage of the classic complement pathway and inhibits thrombin, plasmin, activated Hageman factor (factor XIIa) and kallikrein. Deficiency results in spontaneous activation of C1, leading to consumption of C2 and C4. The functional assay involves the measurement of C1 INH, by its inhibition of the hydrolysis of a substrate ester by C1 esterase. Immunoassay of C1 INH antigen is also available. | Decreased in: Hereditary angioedema (HAE), acquired angioedema. | C1 esterase inhibitor deficiency is an uncommon cause of angioedema. There are three subtypes of HAE. In type 1 (∼85%), both antigenic and functional levels are low; in type 2 (∼15%), antigenic level is normal but functional level is decreased; in type 3 (rare), the C1-INH levels are normal. In some families, type 3 HAE has been linked to mutations in the Hageman factor. Acquired angioedema has been attributed to massive consumption of C1 INH (presumably by tumor or lymphoma-related immune complexes) or to anti-C1 INH autoantibody. When clinical suspicion exists, a serum C4 level screens for HAE. Low levels of C4 are present in all cases during an attack. C1 INH levels are not indicated unless either the C4 level is low or there is a very high clinical suspicion of HAE in a patient with normal C4 during an asymptomatic phase between attacks. In acquired C1 INH deficiency, the C1 level is also significantly decreased (often 10% of normal), whereas in HAE the C1 level is normal or only slightly decreased. Frank MM. Complement disorders and hereditary angioedema. J Allergy Clin Immunol 2010;125(Suppl 2):S262. [PubMed: 20176263] Nagy N et al. New insights into hereditary angio-edema: molecular diagnosis and therapy. Australas J Dermatol. 2010;51:157. [PubMed: 20695852] Zuraw BL et al. Pathogenesis and laboratory diagnosis of hereditary angioedema. Allergy Asthma Proc 2009;30:487. [PubMed: 19843402] |
Calcitonin
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Calcitonin, plasma or serum Males: <8 pg/mL [ng/L] Females: <6 pg/mL [ng/L] Green, SST $$$ Separate serum/plasma from cells ASAP and freeze | Calcitonin is a 32-amino-acid polypeptide hormone secreted by the parafollicular C cells of the thyroid. It decreases osteoclastic bone resorption and lowers serum calcium levels. | Increased in: Medullary thyroid carcinoma, Zollinger-Ellison syndrome, pernicious anemia, pregnancy (at term), newborns, carcinoma (breast, lung, pancreas), leukemia, myeloproliferative disorders, chronic renal failure. | Test is useful to diagnose and monitor medullary thyroid carcinoma, although stimulation tests may be necessary (eg, pentagastrin test). Genetic testing (eg, RET mutation test) is now available for the diagnosis of multiple endocrine neoplasia type II. (MEN II is the most common familial form of medullary thyroid carcinoma.) Ball DW. Medullary thyroid cancer: therapeutic targets and molecular markers. Curr Opin Oncol 2007;19:18. [PubMed: 17133107] Chen H et al. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas 2010;39:775. [PubMed: 20664475] Elisei R. Routine serum calcitonin measurement in the evaluation of thyroid nodules. Best Pract Res Clin Endocrinol Metab 2008;22:941. [PubMed: 19041824] |
Calcium, Serum
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Calcium, serum or plasma (Ca2+) 8.5-10.5 mg/dL [2.1-2.6 mmol/L] Panic: <6.5 or >13.5 mg/dL SST, green $ Prolonged venous stasis during collection causes false increase in serum calcium. | Serum calcium is the sum of ionized calcium plus complexe calcium and calcium bound to proteins (mostly albumin). Level of ionized calcium is regulated by parathyroid hormone and vitamin D. | Increased in: Hyperparathyroidism, malignancies secreting parathyroid hormone-related protein (PTHrP) (especially squamous cell carcinoma of lung and renal cell carcinoma), vitamin D excess, milk-alkali syndrome, multiple myeloma, Paget disease of bone with immobilization, sarcoidosis, other granulomatous disorders, familial hypocalciuria, vitamin Aintoxication, thyrotoxicosis, Addison disease. Drugs: antacids (some), calcium salts, chronic diuretic use (eg, thiazides), lithium, others. Decreased in: Hypoparathyroidism, vitamin D deficiency, renal insufficiency, pseudohypoparathyroidism, magnesium deficiency, hyperphosphatemia, massive transfusion, hypoalbuminemia. | Need to know serum albumin to interpret calcium level. For every decrease in albumin by 1 mg/dL, calcium should be corrected upward by 0.8 mg/dL. In 10% of patients with malignancies, hypercalcemia is attributable to coexistent hyperparathyroidism, suggesting that serum PTH levels should be measured at initial presentation of all hypercalcemic patients (see Figure 9–13). Carlson D. Parathyroid pathology: hyperparathyroidism and parathyroid tumors. Arch Pathol Lab Med 2010;134:1639. [PubMed: 21043817] Habib Z et al. Primary hyperparathyroidism: an update. Curr Opin Endocrinol Diabetes Obes 2010;17:554. [PubMed: 20890202] Lietman SA et al. Hypercalcemia in children and adolescents. Curr Opin Pediatr 2010;22:508. [PubMed: 20601885] |
Calcium, Ionized
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Calcium, ionized, serum or whole blood 4.4-5.4 mg/dL (at pH 7.4) [1.1-1.3 mmol/L] Whole blood specimen must be collected anaerobically and anticoagulated with standardized amounts of heparin. Tourniquet application must be brief. Specimen should be analyzed promptly. SST, green $$ | Calcium circulates in three forms: as free Ca2+ (50–55%), protein-bound to albumin and globulins (40–45%), and as calcium-ligand complexes (5–10%) (with citrate, bicarbonate, lactate, phosphate, and sulfate). Protein binding is highly pH-dependent, and acidosis results in an increased free calcium fraction. Ionized Ca2+ is the form that is physiologically active. Ionized calcium is a more accurate reflection of physiologic status than total calcium in patients with altered serum proteins (renal failure, nephrotic syndrome, multiple myeloma, etc), altered concentrations of calcium-binding ligands, and acid-base disturbances. Measurement of ionized calcium is by ion-selective electrodes. Ionized calcium levels vary inversely with pH, about 0.2 mg/dL per 0.1 pH unit change. | Increased in: ↓ Blood pH. Decreased in: ↑ Blood pH, citrate, EDTA. | Ionized calcium measurements are not needed except in special circumstances, eg, massive blood transfusion, transfusion of whole blood in neonates, liver transplantation, neonatal hypocalcemia, cardiac bypass surgery, and possibly monitoring of patients with secondary hyperparathyroidism from renal failure. Validity of test depends on sample integrity. Ionized calcium normalized to pH 7.4 should be interpreted with caution and along with patient’s acid/base status. See diagnostic algorithms for hypercalcemia and hypocalcemia (Figures 9–13 & 9–15). Morton AR et al. Is the calcium correct? Measuring serum calcium in dialysis patients. Semin Dial 2010;23:283. [PubMed: 20492582] |
Calcium, Urine
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Calcium, urine (UCa) 100–300 mg/24 hr (for persons with average calcium intake, ie, 600–800 mg/d) [2.5–7.5 mmol/24 hr or 2.3–3.3 mmol/12 hr] Urine bottle containing hydrochloric acid $$$ Collect 24-hour urine or 12-hour overnight urine. Refrigerate during collection. | Ordinarily, there is moderate urinary calcium excretion, the amount depending on dietary calcium, parathyroid hormone (PTH) level, and protein intake. Renal calculi occur much more often in those with hyperparathyroidism than in other hypercalcemic states. | Increased in: Hyperparathyroidism, osteolytic bone metastases, myeloma, osteoporosis, vitamin D intoxication, distal RTA, idiopathic hypercalciuria, thyrotoxicosis, Paget disease, Fanconi syndrome, hepatolenticular degeneration, schistosomiasis, sarcoidosis, malignancy (breast, bladder), osteitis deformans, immobilization. Drugs: acetazolamide, calcium salts, cholestyramine, corticosteroids, dihydrotachysterol, initial diuretic use (eg, furosemide), others. Decreased in: Hypoparathyroidism, pseudohypoparathyroidism, rickets, osteomalacia, nephrotic syndrome, acute glomerulonephritis, osteoblastic bone metastases, hypothyroidism, celiac disease, steatorrhea, hypocalciuric hypercalcemia, other causes of hypocalcemia. Drugs: aspirin, bicarbonate, chronic diuretic use (eg, thiazides, chlorthalidone), estrogens, indomethacin, lithium, neomycin, oral contraceptives. | Approximately one third of patients with hyperparathyroidism have normal urine calcium excretion. The extent of calcium excretion can be expressed as a urine calcium (UCa)/urine creatinine (UCr) ratio. Normally, 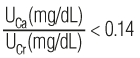 or 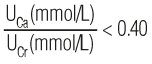 Hypercalciuria is defined as a ratio of >0.20 or >0.57, respectively. Test is useful in the evaluation of renal stones but is not usually needed for the diagnosis of hyperparathyroidism, which can be made using serum calcium (see above) and PTH measurements (see Figure 10–8). It may be useful in hypercalcemic patients to rule out familial hypocalciuric hypercalcemia. In the diagnosis of hypercalciuria, UCa/UCr ratios in random single-voided urine specimens correlate well with 24-hour calcium excretions. Srivastava T et al. Diagnosis and management of hypercalciuria in children. Curr Opin Pediatr 2009;21:214. [PubMed: 19307900] Stechman MJ et al. Genetic causes of hypercalciuric nephrolithiasis. Pediatr Nephrol 2009;24:2321. [PubMed: 18446382] Tasca A et al. Bone disease in patients with primary hypercalciuria and calciumnephrolithiasis. Urology 2009;74:22. [PubMed: 19428073] |
Carbon Dioxide, Partial Pressure
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Carbon dioxide, partial pressure (PCO2), whole blood Arterial: 32–48 mm Hg (4.26–6.38 kPa) Heparinized syringe $$$ Specimen must be collected in heparinized syringe and immediately transported on ice to lab without exposure to air. | The partial pressure of carbon dioxide in arterial blood (PCO2) provides important information with regard to adequacy of ventilation, and acid–base status. | Increased in: Respiratory acidosis: decreased alveolar ventilation (eg, COPD, respiratory depressants), neuromuscular diseases (eg, myasthenia gravis). Decreased in: Respiratory alkalosis: hyperventilation (eg, anxiety), sepsis, liver disease, fever, early salicylate poisoning, and excessive artificial ventilation. | See laboratory characteristics of acid–base disturbances (Figure 9–1, Table 8–1). Kraut JA et al. Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol 2010;6:274. [PubMed: 20308999] Zhou W et al. Hypercapnia and hypocapnia in neonates. World J Pediatr 2008;4:192. [PubMed: 18822927] |
Carbon Dioxide, (Total Bicarbonate)
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Carbon dioxide (total bicarbonate), serum or plasma 22–28 meq/L [mmol/L] Panic: <15 or >40 meq/L [mmol/L] SST, green $ | Bicarbonate-carbonic acid buffer is one of the most important buffer systems in maintaining normal body fluid pH. Total carbon dioxide (CO2) is measured as the sum of bicarbonate (HCO3−) concentration and dissolved CO2 (carbonic acid concentration plus dissolved free CO2). Total CO2 measurements use either electrode-based or enzymatic methods. Because HCO3− makes up 90–95% of the total CO2 content, total CO2 is a useful surrogate for HCO3− concentration. | Increased in: Primary metabolic alkalosis, compensated respiratory acidosis, volume contraction, mineralocorticoid excess, congenital chloridorrhea. Drugs: diuretics (eg, thiazide, furosemide). Decreased in: Metabolic acidosis, compensated respiratory alkalosis. Fanconi syndrome, volume overload. Drugs: acetazolamide, outdated tetracycline. | Total CO2 determination is indicated for all seriously ill patients on admission. Simultaneous measurement of HCO3, pH, and PCO2 is required to fully characterize a patient’s acid–base status. See Acid–base disturbance (Table 8–1; Figure 9–1). Kraut JA et al. Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol 2010;6:274. [PubMed: 20308999] |
Carboxyhemoglobin
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Carboxyhemoglobin, whole blood (COHb) <9% [<0.09] Blood gas syringe or green $$ Specimen should be collected before treatment with oxygen is started. Do not remove stopper or cap. | Carbon monoxide (CO) is an odorless and nonirritating gas formed by hydrocarbon combustion. CO binds to hemoglobin with much greater affinity (∼240 times) than oxygen, forming carboxyhemoglobin (COHb) and resulting in impaired oxygen transport/delivery and utilization. CO can also precipitate an inflammatory cascade that results in CNS lipid peroxidation and delayed neurologic sequelae. | Increased in: Carbon monoxide poisoning, exposure to automobile exhaust, smoke from fires, coal gas, and defective furnaces. Cigarette smokers can have up to 9% carboxyhemoglobin, while nonsmokers have <2%. | Laboratory CO-oximetry is widely available for rapid evaluation of CO poisoning. Toxic effects (headache, dizziness, nausea, confusion, and/or unconsciousness) occur if the COHb level is >10–15%. Levels > 40% may be fatal if not treated immediately with oxygen. PO2 is usually normal in CO poisoning. Kealey GP. Carbon monoxide toxicity. J Burn Care Res 2009;30(1):146. [PubMed: 19060737] Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med 2009;360:1217. |
Carcinoembryonic Antigen
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Carcinoembryonic antigen, serum (CEA) <2.5 ng/mL [mcg/L] SST $$ | CEA is an oncofetal antigen, a glycoprotein associated with certain malignancies, particularly epithelial tumors (eg, colorectal cancer, pancreatic cancer, etc). | Increased in: Colorectal cancer (72%), lung cancer (76%), pancreatic cancer (91%), stomach cancer (61%), cigarette smokers, benign acute (50%) and chronic (90%) liver disease, benign GI disease (peptic ulcer, pancreatitis, colitis). Elevations >20 ng/mL are generally associated with malignancy. For breast cancer recurrence (using 5 ng/mL cutoff), sensitivity is 44.4% and specificity, 95.5%. | Screening: Test is not sensitive or specific enough to be useful in cancer screening. CEA levels should be used in conjunction with clinical evaluation and other diagnostic procedures. Monitoring after surgery: Test is used to detect recurrence of colorectal cancer after surgery (elevated CEA levels suggest recurrence 3–6 months before other clinical indicators), although such monitoring has not yet been shown to improve survival rates. If monitoring is done, the same assay method must be used consistently to eliminate any method-dependent variability. Holt A et al. Surveillance with serial serum carcinoembryonic levels detect colorectal cancer recurrences in patients who are initial nonsecretors. Am Surg 2010;76:1100. [PubMed: 21105619] Tan E et al. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol 2009;18:15. [PubMed: 18619834] |
CD4 Cell Count
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
CD4 cell count, absolute, whole blood CD4: 359–1725 cells/mcL (29–61%) Lavender, yellow $$$ For an absolute CD4 count, order T-cell subsets and a CBC with differential. | Lymphocyte identification depends on specific cell surface CD (clusters of differentiation) antigens, which can be detected by flow cytometry analysis using monoclonal antibodies. The CD4 cells (helper T cells) express both CD3 (a pan–T-cell marker) and CD4. The CD8 cells (suppressor T cells) express both CD3 and CD8. CD4 cell levels are a criterion for categorizing HIV-related clinical conditions by CDC’s classification system for HIV infection. The measurement of CD4 cell levels has been used to establish decision points for initiating prophylaxis and antiviral therapy and to monitor the efficacy of treatment. It has been recommended that CD4 cell levels be monitored every 3–6 months in all HIV-infected persons. | Increased in: Rheumatoid arthritis, type 1 diabetes mellitus, SLE without renal disease, primary biliary cirrhosis, atopic dermatitis, Sézary syndrome, psoriasis, chronic autoimmune hepatitis. Decreased in: AIDS/HIV infection, SLE with renal disease, acute cytomegalovirus (CMV) infection, burns, graft-versus-host disease, sunburn, myelodysplastic syndromes, acute lymphoblastic leukemia in remission, recovery from bone marrow transplantation, herpes infection, infectious mononucleosis, measles, ataxia-telangiectasia, vigorous exercise. | During HIV infection, antiviral therapy is often initiated when the absolute CD4 count drops below 500 cells/mcL. When the absolute CD4 count drops below 200 cells/mcL, therapeutic prophylaxis against Pneumocystis jiroveci pneumonia (PCP) and other opportunistic infections may be initiated. When the absolute CD4 count drops below 100 cells/mcL, prophylaxis against Mycobacterium avium complex is recommended. For longitudinal studies involving serial monitoring, specimen collections should be performed at the same time of day. Cambiano V et al. ‘Test-and-treat’: the end of the HIV epidemic? Curr Opin Infect Dis 2011;24:19. [PubMed: 21157329] Jain V et al. When to start antiretroviral therapy. Curr HIV/AIDS Rep 2010;7:60. [PubMed: 20425559] Sabin CA et al. Should HIV therapy be started at a CD4 cell count above 350 cells/microl in asymptomatic HIV-1-infected patients? Curr Opin Infect Dis 2009;22:191. [PubMed: 19283914] |
Celiac Disease, Serologic Testing
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Celiac disease serologic testing, serum Negative SST, red <SPAN role=presentation tabIndex=0 id=MathJax-Element-10-Frame class=MathJax style="POSITION: relative" data-mathml='‘> | Celiac disease (gluten-sensitive enteropathy) is associated with a variety of autoantibodies, including tissue transglutaminase (tTG), endomysial, and deamidated gliadin antibodies. Although the IgA isotype of these antibodies usually predominates in celiac disease, individuals may also produce IgG isotypes, particularly those who are IgA deficient. The most sensitive and specific serologic tests are tTG and deamidated gliadin antibodies. For patients with selective IgA deficiency, serum tTG, gliadin (deamidated), and endomysial autoantibodies of IgG type should be tested. | Positive in: Celiac disease (90% cases have 1 or more of the 3 autoantibodies). | Useful for evaluating patients suspected of having celiac disease, including patients with compatible symptoms, those with atypical symptoms, and those at increased risk (family history and/or positivity for DQ2 and/or DQ8). Those with positive laboratory results should then be referred for small intestinal biopsy to confirm the diagnosis. Genetic susceptibility of celiac disease is related to specific HLA markers, ie, HLA DQ2 and/or DQ8. Ensari A. Gluten-sensitive enteropathy (celiac disease): controversies in diagnosis and classification. Arch Pathol Lab Med 2010;134:826. [PubMed: 20524861] Green PH et al. Medical progress: Celiac disease. N Engl J Med 2007;357:1731. [PubMed: 17960014] Leffler DA et al. Update on serologic testing in celiac disease. Am J Gastroenterol 2010;105:2520. [PubMed: 21131921] |
Centromere Antibody
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Centromere antibody, serum (ACA) Negative SST $$ | Centromere antibodies (ACA) are antibodies to nuclear proteins, specifically CENP-A, B, and C. The CENP-B is the primary autoantigen and is recognized by all sera that contain ACA. Presence of ACA predicts a favorable prognosis for systemic sclerosis. Both immunofluorescent antibody testing (IFA)- and enzyme-linked immunosorbent assay (ELISA)-based assays are available for ACA detection. | Positive in: CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) (80–90%), diffuse scleroderma (5–10%), Raynaud disease (20–30%). | In patients with connective tissue disease, the predictive value of a positive test is >95% for scleroderma or related disease (CREST syndrome, Raynaud disease). Diagnosis of CREST syndrome is made clinically. The presence of detectable ACA may antedate the development of clinical CREST syndrome by several years. ACA is also present in a small percentage of patients with primary biliary cirrhosis, rheumatoid arthritis and systemic lupus erythematosus. (See also Autoantibodies, Table 8–6.) Hamaguchi Y. Autoantibody profiles in systemic sclerosis: predictive value for clinical evaluation and prognosis. J Dermatol 2010;37:42. [PubMed: 20175839] |
Ceruloplasmin, Serum
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Ceruloplasmin, serum or plasma 20–40 mg/dL [200–500 mg/L] (age-dependent) SST, PPT, green (fasting specimen is preferred) $$ | Ceruloplasmin, a 120,000–160,000 MW α2-glycoprotein with oxidase activity synthesized by the liver, is the main (95%) copper-carrying protein in human serum. Any failure during its synthesis whereby copper cannot be incorporated into ceruloplasmin results in secretion of an apoceruloplasmin. The apo form has a short half-life and is rapidly metabolized, leading to reduced serum level of ceruloplasmin. | Increased in: Acute and chronic inflammation, pregnancy. Drugs: oral contraceptives, phenytoin. Decreased in: Wilson disease (hepatolenticular degeneration) (95%), CNS disease other than Wilson (15%), liver disease other than Wilson (23%), malabsorption (enteropathy), malnutrition, primary biliary cirrhosis, nephrotic syndrome, severe copper deficiency, Menkes disease (X-linked inherited copper deficiency), hereditary aceruloplasminemia. | Serum ceruloplasmin level and slit-lamp examination for Kayser-Fleischer rings are initial recommended tests for diagnosis of Wilson disease. Slit-lamp exam is only 50–60% sensitive in patients without neurologic symptoms. Equivocal cases may need 24-hour urinary copper excretion, liver copper measurement, and/or detection of ATP7B gene mutations. Serum/plasma copper level is rarely indicated. Serum/plasma ceruloplasmin for diagnosis of Wilson disease is not reliable in asymptomatic patients. Mak CM et al. Diagnosis of Wilson’s disease: a comprehensive review. Crit Rev Clin Lab Sci 2008;45:263. [PubMed: 18568852] Nicastro E et al. Re-evaluation of the diagnostic criteria for Wilson disease in children with mild liver disease. Hepatology 2010;52:1948. [PubMed: 20967755] |
Chloride
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Chloride, serum or plasma (Cl−) 98–107 meq/L [mmol/L] SST, green $ | Chloride, the principal inorganic anion of extracellular fluid, is important in maintaining proper body water distribution, osmotic pressure, and normal acid–base balance. If chloride is lost (as HCl or NH4Cl), alkalosis ensues; if chloride is ingested or retained, acidosis ensues. | Increased in: Renal failure, nephrotic syndrome, renal tubular acidosis, dehydration, overtreatment with saline, hyperparathyroidism, diabetes insipidus, metabolic acidosis from diarrhea (loss of HCO3−), respiratory alkalosis, hyperadrenocorticism. Drugs: acetazolamide (hyperchloremic acidosis), androgens, hydrochlorothiazide, salicylates (intoxication). Decreased in: Vomiting, diarrhea, gastrointestinal suction, renal failure combined with salt deprivation, over-treatment with diuretics, chronic respiratory acidosis, diabetic ketoacidosis, excessive sweating, SIADH, salt-losing nephropathy, acute intermittent porphyria, water intoxication, expansion of extracellular fluid volume, adrenal insufficiency, hyperaldosteronism, metabolic alkalosis. Drugs: chronic laxative or bicarbonate ingestion, corticosteroids, diuretics. | Test is helpful in assessing normal and increased anion gap metabolic acidosis. It is somewhat helpful in distinguishing hypercalcemia due to primary hyperparathyroidism (high serum chloride) from that due to malignancy (normal serum chloride). Yunos NM et al. Bench-to-bedside review: chloride in critical illness. Crit Care 2010;14:226. [PubMed: 20663180] |
Cholesterol
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Cholesterol, serum or plasma Desirable: <200 mg/dL [<5.2 mmol/L] Borderline: 200–239 mg/dL [5.2–6.1 mmol/L] High risk: >240 mg/dL [>6.2 mmol/L] SST, PPT, green $ Fasting specimen is required for LDL-C determination. HDL-C and total cholesterol can be measured with nonfasting specimen. | Cholesterol level is determined by lipid metabolism, which is in turn influenced by heredity, diet, and liver, kidney, thyroid, and other endocrine organ functions. Screening for total cholesterol (TC) may be done with nonfasting specimens, but a complete lipoprotein profile or LDL cholesterol (LDL-C) determination must be performed on fasting specimens. TC, triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) are directly measured. Although methods have been developed for direct LDL-C measurement, in practice, LDL-C is often indirectly determined by use of the Friedewald equation: [LDL-C] = [TC] − [HDL-C] − [TG/5] Note that this calculation is not valid for specimens having TG >400 mg/dL [>4.52 mmol/L], for patients with type III hyperlipoproteinemia or chylomicronemia, or nonfasting specimens. | Increased in: Primary disorders: polygenic hypercholesterolemia, familial hypercholesterolemia (deficiency of LDL receptors), familial combined hyperlipidemia, familial dysbetalipoproteinemia. Secondary disorders: hypothyroidism, uncontrolled diabetes mellitus, nephrotic syndrome, biliary obstruction, anorexia nervosa, hepatocellular carcinoma, Cushing syndrome, acute intermittent porphyria. Drugs: corticosteroids. Decreased in: Severe liver disease (acute hepatitis, cirrhosis, malignancy), hyperthyroidism, severe acute or chronic illness, malnutrition, malabsorption (eg, HIV), extensive burns, familial (Gaucher disease, Tangier disease), abetalipoproteinemia, intestinal lymphangiectasia. | Coronary heart disease (CHD) risk depends on the ratio of total cholesterol to HDL cholesterol. The ratio of LDL to HDL cholesterol has similar predictive ability. Treatment decisions should be based on absolute CHD risk. The risk reduction is proportional to the reduction in LDL cholesterol achieved with treatment. The National Cholesterol Education Program (NCEP) expert panel has published clinical recommendations. According to NCEP guidelines, HDL-C <40 mg/dL is a risk factor for coronary heart disease (CHD), and HDL-C ≥60 mg/dL is a “negative” risk factor. In addition, there is a direct relation between LDL-C and the incidence of CHD. Treatment decisions and therapeutic goals are primarily based on LDL-C concentrations. The recommended LDL-C intervention goals are <100 mg/dL for high-risk patients (eg, patients with CHD), <130 mg/dL for moderate-risk patients (≥2 risk factors), and <160 mg/dL for low-risk patients (no or 1 risk factor). See Table 8–12 for risk factor assessment for CHD. Alwaili K et al. High-density lipoproteins and cardiovascular disease: 2010 update. Expert Rev Cardiovasc Ther 2010;8:413. [PubMed: 20222819] Baumer JH et al. Hypercholesterolaemia in children guidelines review. Arch Dis Child Educ Pract Ed 2009;94:84. [PubMed: 19460897] Viera AJ et al. Global risk of coronary heart disease: assessment and application. Am Fam Physician 2010;82:265. [PubMed: 20672791] |
Clostridium difficile Enterotoxin
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Clostridium difficile toxins, stool Negative Urine or stool container for collection of diarrheal (unformed) stool $$$ Must be tested within 12 hours of collection because toxin (B) is labile. | Clostridium difficile, a motile, gram-positive rod, is the major recognized agent of antibiotic-associated diarrhea, which is toxigenic in origin (see Antibiotic-associated colitis, Chapter 5). There are two toxins (A and B) produced by C. difficile; toxin A is an enterotoxin and toxin B is a cytotoxin. Cytotoxicity assay performed in cell culture is used to detect the cytopathic effect of the toxins, whose identity is confirmed by neutralization with specific antitoxins. The assay sensitivity and specificity are 95% and 90%, respectively. However, the assay is expensive and requires 24–48 hours and is thus not clinically practical. Toxin A (more weakly cytopathic in cell culture) is enterotoxic and produces enteric disease. Toxin B (more easily detected in standard cell culture assays) fails to produce intestinal disease. | Positive in: Antibiotic-associated diarrhea (15–25%), antibiotic-associated colitis (50–75%), and pseudomembranous colitis (90–100%). About 3% of healthy adults and 10–20% of hospitalized patients have C. difficile in their colonic flora. There is also a high carrier rate of C. difficile and its toxin in healthy neonates. | Rapid enzyme immunoassay (EIA) (2–4 hour) tests for toxin A or toxins A and B have been used as an alternative to the cytotoxicity assay but are less sensitive and thus suboptimal. New guidelines recommend a two-step testing process, which includes an initial screening of stool samples with a rapid immunoassay for glutamate dehydrogenase (GDH), a common enzyme produced by C. difficile. A negative GDH assay effectively rules out infection, while a positive assay requires confirmation with a more specific assay, ie, the cell cytotoxicity assay or toxigenic culture. PCR assay that amplifies the genes responsible for C. difficile toxins may ultimately provide a more rapid, sensitive and specific test. Repeat testing during the same episode of diarrhea is of limited value and should be discouraged. Direct visualization with histopathologic examination of pseudomembranes on lower gastrointestinal endoscopy only detects 50–55% of C. difficile cases. Cohen SH et al. Clinical practice guidelines for Clostridium difficile in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431. [PubMed: 20307191] Curry S. Clostridium difficile. Clin Lab Med 2010;30(1):329. [PubMed: 20513554] |
Coccidioides Antibodies
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Coccidioides antibodies, serum or CSF Negative SST or red (serum); glass or plastic (CSF) $$ | Screens for presence of antibodies to Coccidioides immitis. Some centers use the mycelial-phase antigen, coccidioidin, to detect antibody. IgM antibodies appear early in disease in 75% of patients, begin to decrease after week 3, and are rarely seen after 5 months. They may persist in disseminated cases, usually in immunocompromised patients. IgG antibodies appear later in the course of the disease. Meningeal disease may have negative serum IgG and require CSF IgG antibody titers. | Positive in: Infection by coccidioides (90%). Negative in: Coccidioidin skin testing, many patients with chronic cavitary coccidioides; 5% of meningeal coccidioides is negative by CSF complement fixation (CF) test. | Diagnosis is based on culture and serologic testing. Precipitin (immunodiffusion) and CF tests detect 90% of primary symptomatic cases. Precipitin test (for IgM and IgG antibodies) is most effective in detecting early primary infection or an exacerbation of existing disease. Test is diagnostic but not prognostic. CF test (for IgG antibody) becomes positive later than precipitin test, and titers can be used to assess severity of infection. Titers rise as the disease progresses and decline as the patient improves. ELISA-based test is also available; data suggest good test performance characteristics. Ampel NM. New perspectives on coccidioidomycosis. Proc Am Thorac Soc 2010;7:181. [PubMed: 20463246] Parish JM et al. Coccidioidomycosis. Mayo Clin Proc 2008;83:343. [PubMed: 18316002] |
Cold Agglutinins
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Cold agglutinins, serum <1:32 titer Red, SST $$ Specimen should be kept at 37°C before separation from cells. | Cold agglutinins are IgM (rarely IgG or IgA) autoantibodies that are capable of agglutinating red blood cells (RBCs) at temperature below 35°C (strongly at 4°C, weakly at 24°C, and weakly or not at all at 37°C). Cold agglutinins can be monoclonal or polyclonal, and have been associated with various diseases, particularly infections, neoplasms, and collagen vascular diseases. Cold agglutinins are not necessarily pathologic, and may be detected in asymptomatic individuals during routine blood typing and crossmatching. If the agglutination is not reversible after incubation at 37°C, then the reaction is not due to cold agglutinins. | Increased in: Chronic cold agglutinin disease, lymphoproliferative disorders (eg, Waldenström macro-globulinemia, chronic lymphocytic leukemia), autoimmune hemolytic anemia, myeloma, collagen-vascular diseases, Mycoplasma pneumoniae pneumonia, infectious mononucleosis, mumps orchitis, cytomegalovirus, listeriosis, tropical diseases (eg, trypanosomiasis, malaria). | Patients with cold agglutinins develop anti-I or anti-i antibodies which are usually of the IgM class and react with adult human RBCs at temperatures below 35°C, resulting in agglutination. In Mycoplasma pneumonia, titers of anti-I rise late in the first week or during the second week, are maximal at 3–4 weeks after onset, and then disappear rapidly. A rise in cold agglutinin antibody titer is suggestive of recent mycoplasma infection. Berentsen S. Cold agglutinin-mediated autoimmune hemolytic anemia in Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma 2009;9:110. [PubMed: 19362990] Mayer B et al. Mixed-type autoimmune hemolytic anemia: differential diagnosis and a critical review of reported cases. Transfusion 2008;48:2229. [PubMed: 18564390] |
Complement C3
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Complement C3, serum 64–166 mg/dL [640–1660 mg/L] SST $$ | The classic and alternative complement pathways converge at the C3 step in the complement cascade. Low levels indicate activation by one or both pathways. Most diseases with immune complexes show decreased C3 levels. Test is usually performed as an immunoassay (by radial immunodiffusion or nephelometry). | Increased in: Many inflammatory conditions as an acute-phase reactant, active phase of rheumatic diseases (eg, rheumatoid arthritis, SLE), acute viral hepatitis, myocardial infarction, cancer, diabetes mellitus, pregnancy, sarcoidosis, amyloidosis, thyroiditis. Decreased by: Decreased synthesis (protein malnutrition, congenital deficiency, severe liver disease), increased catabolism (immune complex disease, membranoproliferative glomerulonephritis [75%], SLE, Sjögren syndrome, rheumatoid arthritis, DIC, paroxysmal nocturnal hemoglobinuria, autoimmune hemolytic anemia, gram-negative bacteremia), increased loss (burns, gastroenteropathies). | Complement C3 levels may be useful in following the activity of immune complex diseases. The best test to detect inherited deficiencies is CH50 (complement activity assay). Carroll MC. Complement and humoral immunity. Vaccine 2008;26(Suppl 8):128. [PubMed: 19388161] Reis E et al. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand J Immunol 2006;63:155. [PubMed: 16499568] |
Complement C4
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Complement C4, serum 15–45 mg/dL [150–450 mg/dL] SST $$ | C4 is a component of the classic complement pathway. Depressed levels usually indicate classic pathway activation. Test is usually performed as an immunoassay and not a functional assay. | Increased in: Various malignancies (not clinically useful). Decreased by: Decreased synthesis (congenital deficiency), increased catabolism (SLE, rheumatoid arthritis, proliferative glomerulonephritis, hereditary angioedema (HAE)), and increased loss (burns, protein-losing enteropathies). | Low C4 accompanies acute attacks of HAE, and C4 is used as a first-line test for the disease. C1 esterase inhibitor levels are not indicated for the evaluation of HAE unless C4 is low. Congenital C4 deficiency occurs with an SLE-like syndrome. Arason GJ et al. Primary immunodeficiency and autoimmunity: lessons from human diseases. Scand J Immunol 2010;71:317. [PubMed: 20500682] Breda L et al. Laboratory tests in the diagnosis and follow-up of pediatric rheumatic diseases: an update. Semin Arthritis Rheum 2010;40:53. [PubMed: 19246077] Lipsker D et al. Cutaneous manifestations of complement deficiencies. Lupus 2010;19:1096. [PubMed: 20693203] |
Complement CH50
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Complement CH50, serum (CH50) 22–40 U/mL (laboratory-specific) Red $$$ | The quantitative assay of hemolytic complement activity depends on the ability of the classic complement pathway to induce hemolysis of red cells sensitized with optimal amounts of anti-red cell antibodies. For precise titrations of hemolytic complement, the dilution of serum that lyses 50% of the indicator red cells is determined as the CH50. This arbitrary unit depends on the conditions of the assay and is therefore laboratory-specific. | Decreased with: >50–80% deficiency of classic pathway complement components (congenital or acquired deficiencies). Normal in: Deficiencies of the alternative pathway complement components. | This is a functional assay of biologic activity. Sensitivity to decreased levels of complement components depends on exactly how the test is performed. It is used to detect congenital and acquired severe deficiency disorders of the classic complement pathway. Botto M et al. Complement in human diseases: lessons from complement deficiencies. Mol Immunol 2009;46:2774. [PubMed: 19481265] Chen M et al. The complement system in systemic autoimmune disease. J Autoimmun 2010;34:J276. [PubMed: 20005073] Pettigrew HD et al. Clinical significance of complement deficiencies. Ann N Y Acad Sci 2009;1173:108. [PubMed: 19758139] |
Complete Blood Cell Count
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Complete blood cell count (CBC), blood Refer to individual test for reference range Lavender $ | The CBC consists of a panel of tests that examines whole blood and includes the following: total white blood cell count (WBC, ×103/mcL) and white blood cell differential (%), red blood cell count (RBC, × 106/mcL), hemoglobin concentration (Hb, g/L), hematocrit (Hct, %), platelet count (Plt, 103/mcL), red cell indices including mean corpuscular volume (MCV, fL), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC, g/L), and red cell distribution width (RDW, %). Several new CBC parameters are being introduced, including nucleated red blood cells, immature granulocytes, immature reticulocyte fraction, immature platelet fraction, red cell fragments as well as new parameters for detection of functional iron deficiency. Automated laboratory hematology analyzers are widely available. The basic principles used for the cell counting and white cell differential are instrument-dependent. | Refer to individual test for detailed information. Also see Table 8–31 for white cell count and differential. | The CBC provides important information about the types and numbers of cells in the blood, especially red cells, white cells, and platelets. It helps in evaluating symptoms (eg, weakness, fatigue, fever or bruising), diagnosing conditions/ diseases (eg, anemia, infection, leukemia, and many other disorders), and determining the stages of a particular disease (eg, leukemia). Hct, MCH, and MCHC are typically calculated from RBC, Hb, and MCV. If significantly abnormal CBC values are obtained, a peripheral blood smear should be prepared and examined (eg, red cell morphology, WBC differential, platelet count estimation, identification of immature and malignant cells). Briggs C. Quality counts: new parameters in blood cell counting. Int J Lab Hematol 2009;31:277. [PubMed: 19452619] Milcic TL. The complete blood count. Neonatal Netw 2010;29:109. [PubMed: 20211833] |
Cortisol
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Cortisol, plasma or serum 8:00 AM: 5–20 mcg/dL [140–550 nmol/L] SST, lavender, or green $$ | Release of corticotropin-releasing factor (CRF) from the hypothalamus stimulates release of ACTH from the pituitary, which in turn stimulates release of cortisol from the adrenal. Cortisol provides negative feedback to this system. Test measures both free cortisol and cortisol bound to cortisol-binding globulin (CBG). Morning levels are higher than evening levels. | Increased in: Cushing syndrome, acute illness, surgery, trauma, septic shock, depression, anxiety, alcoholism, starvation, chronic renal failure, increased CBG (congenital, pregnancy, estrogen therapy). Decreased in: Addison disease; decreased CBG (congenital, liver disease, nephrotic syndrome). | Cortisol levels are useful only in the context of standardized suppression or stimulation tests. See Cosyntropin stimulation test and Dexamethasone suppression tests for details. Circadian fluctuations in cortisol levels limit usefulness of single measurements. Analysis of diurnal variation of cortisol is not useful diagnostically. Anagnostis P et al. Clinical review: the pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 2009;94:2692. [PubMed: 19470627] Newell-Price J. Diagnosis/differential diagnosis of Cushing’s syndrome: a review of best practice. Best Pract Res Clin Endocrinol Metab 2009;23(Suppl 1):S5. [PubMed: 20129193] Pecori Giraldi F. Recent challenges in the diagnosis of Cushing’s syndrome. Horm Res 2009;71(Suppl 1):123. [PubMed: 19153521] Satre TJ et al. Clinical inquiries. What’s the most practical way to rule out adrenal insufficiency? J Fam Pract 2009;58:281a-b. [PubMed: 19442385] Wallace I et al. The diagnosis and investigation of adrenal insufficiency in adults. Ann Clin Biochem 2009;46(Pt 5):351. [PubMed: 19675057] |
Cortisol (Urinary Free)
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Cortisol (urinary free), urine 10–110 mcg/24 hr [30–300 nmol/d] Urine bottle containing boric acid. $$$ Collect 24-hour urine. | Urinary free cortisol measurement is useful in the initial evaluation of suspected Cushing syndrome (see Cushing syndrome algorithm, Figure 9–8). | Increased in: Cushing syndrome, acute illness, stress. Not increased in: Obesity. | Urinary free cortisol is the initial diagnostic test of choice for Cushing syndrome. Not useful for the diagnosis of adrenal insufficiency. A shorter (12-hour) overnight collection and measurement of the ratio of urine-free cortisol to urine creatinine appears to perform nearly as well as a 24-hour collection for urine-free cortisol. Boscaro M et al. Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab 2009;94:3121. [PubMed: 19734443] Carroll TB et al. The diagnosis of Cushing’s syndrome. Rev Endocr Metab Disord 2010;11:147. [PubMed: 20821267] Newell-Price J. Diagnosis/differential diagnosis of Cushing’s syndrome: a review of best practice. Best Pract Res Clin Endocrinol Metab 2009;23(Suppl 1):S5. [PubMed: 20129193] |
Cosyntropin Stimulation Test
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Cosyntropin stimulation test, serum or plasma SST, green, or lavender $$$ First draw a cortisol level. Then administer cosyntropin (1 mcg or 250 mcg IV). Draw another cortisol level in 30 minutes. | Cosyntropin (synthetic ACTH preparation) stimulates the adrenal to release cortisol. A normal response is a doubling of basal levels or an increment of 7 mcg/dL (200 nmol/L) to a level above 18 mcg/dL (>504 nmol/L). A poor cortisol response to cosyntropin indicates adrenal insufficiency (see Adrenocortical insufficiency algorithm, Figure 9–3). | Decreased in: Adrenal insufficiency, pituitary insufficiency, AIDS. | Test does not distinguish primary from secondary (pituitary) adrenal insufficiency, because in secondary adrenal insufficiency the atrophic adrenal may be unresponsive to cosyntropin. Test may not reliably detect pituitary insufficiency. Metyrapone test may be useful to assess the pituitary-adrenal axis. AIDS patients with adrenal insufficiency may have normal ACTH stimulation tests. Fleseriu M et al. “Relative” adrenal insufficiency in critical illness. Endocr Pract 2009;15:632. [PubMed: 19625244] Magnotti M et al. Diagnosing adrenal insufficiency: which test is best–the 1-microg or the 250-microg test? Endocr Pract 2008;14:233. [PubMed: 18308665] Satre TJ et al. Clinical inquiries. What’s the most practical way to rule out adrenal insufficiency? J Fam Pract 2009;58:281a-b. [PubMed: 19442385] |
Creatine Kinase
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Creatine kinase, serum or plasma (CK) 32–267 IU/L [0.53–4.45 mckat/L] (method-dependent) SST, PPT, green $ | Creatine kinase splits creatine phosphate in the presence of ADP to yield creatine and ATP. Skeletal muscle, myocardium, and brain are rich in the enzyme. CK is released by tissue damage. | Increased in: Myocardial infarction (MI), myocarditis, muscle trauma, rhabdomyolysis, muscular dystrophy, polymyositis, severe muscular exertion, malignant hyperthermia, hypothyroidism, cerebral infarction, surgery, Reye syndrome, tetanus, generalized convulsions, alcoholism, IM injections, DC countershock. Drugs: clofibrate, HMG-CoA reductase inhibitors. | CK is as sensitive a test as aldolase for muscle damage, so aldolase is not needed for this condition. During an MI, serum CK level rises rapidly (within 3–5 hours); elevation persists for 2–3 days post-MI. Total CK is not specific enough for use in diagnosis of MI, but a normal total CK has a high negative predictive value. A more specific test is needed for diagnosis of MI or acute coronary syndrome (eg, CK-MB, now largely replaced by cardiac troponin I). Brancaccio P et al. Biochemical markers of muscular damage. Clin Chem Lab Med 2010;48:757. [PubMed: 20518645] Cervellin G et al. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med 2010;48:749. [PubMed: 20298139] Ristagno G et al. Biomarkers of myocardial injury after cardiac arrest or myocardial ischemia. Front Biosci (Schol Ed) 2010;2:373. [PubMed: 20036954] |
Creatine Kinase MB
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Creatine kinase MB, enzyme activity (CK-MB) <16 IU/L [<0.27 mckat/L] or <4% of total CK or <7 mcg/L mass units (laboratory-specific) SST, PPT, green $$ | CK consists of three isoenzymes, made up of 2 subunits, M and B. The fraction with the greatest electrophoretic mobility is CK1 (BB), CK2 (MB) is intermediate, and CK3 (MM) moves slowest toward the anode. Skeletal muscle is characterized by isoenzyme MM and brain by isoenzyme BB. Myocardium has approximately 40% MB isoenzyme. Assay techniques include isoenzyme separation by electrophoresis (isoenzyme activity units) or by immunoassay using antibody specific for MB fraction (mass units). | Increased in: Myocardial infarction, cardiac trauma, certain muscular dystrophies, and polymyositis. Slight persistent elevation reported in a few patients on hemodialysis. | CK-MB is a relatively specific test for MI. It appears in serum approximately 4 hours after infarction, peaks at 12–24 hours, and declines over 48–72 hours. CK-MB mass concentration is a sensitive marker of MI within 4–12 hours after infarction. Because cardiac troponins are now the markers of choice for the diagnosis of acute MI, high sensitivity cardiac troponin I test has largely replaced the conventional CK-MB assay. Measurement of CK-MB remains useful in evaluating patients who are already troponin positive and have recurrent chest pain and in follow-up of patients who are status post interventional procedures. Estimation of CK-MM and CK-BB is not clinically useful. Use total CK. McLean AS et al. Bench-to-bedside review: the value of cardiac biomarkers in the intensive care patient. Crit Care 2008;12:215. [PubMed: 18557993] Saenger AK. A tale of two biomarkers: the use of troponin and CK-MB in contemporary practice. Clin Lab Sci 2010;23:134. [PubMed: 20734885] |
Creatinine
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Creatinine, serum or plasma (Cr) 0.6–1.2 mg/dL [50–100 mcmol/L] SST, PPT, green $ | Endogenous creatinine is excreted by filtration through the glomerulus and by tubular secretion. Creatinine clearance is an acceptable clinical measure of glomerular filtration rate (GFR), although it sometimes overestimates GFR (eg, in cirrhosis). For each 50% reduction in GFR, serum creatinine approximately doubles. | Increased in: Acute or chronic renal failure, urinary tract obstruction, nephrotoxic drugs, hypothyroidism. Decreased in: Reduced muscle mass, cachexia, aging. | In the alkaline picrate method, substances other than Cr (eg, acetoacetate, acetone, β-hydroxybutyrate, α-ketoglutarate, pyruvate, glucose) may give falsely high results. Therefore, patients with diabetic ketoacidosis may have spuriously elevated Cr. Cephalosporins may spuriously increase or decrease Cr measurement. Increased bilirubin may spuriously decrease Cr. Chronic renal insufficiency may be underrecognized. Age, male sex, and black race are predictors of kidney disease. Serum creatinine levels frequently do not reflect decreased renal function because creatinine production rate is decreased with reduced lean body mass. Increased intravascular volume and increased volume of distribution associated with anasarca may also mask decreased renal function by reducing serum creatinine levels. See glomerular filtration rate, estimated (eGFR). Fliser D. Assessment of renal function in elderly patients. Curr Opin Nephrol Hypertens 2008;17:604. [PubMed: 18941354] Pottel H et al. On the relationship between glomerular filtration rate and serum creatinine in children. Pediatr Nephrol 2010;25:927. [PubMed: 20012996] Wu I et al. Screening for kidney diseases: older measures versus novel biomarkers. Clin J Am Soc Nephrol 2008;3:1895. [PubMed: 18922990] |
Creatinine Clearance
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Creatinine clearance (ClCr) Adults: 90–130 mL/min/1.73 m2 BSA $$ Collect carefully timed 24-hour urine and simultaneous serum/plasma creatinine sample. Record patient’s weight and height. | Widely used test of glomerular filtration rate. Theoretically reliable, but often compromised by incomplete urine collection. Creatinine clearance is calculated from measurement of urine creatinine (UCr [mg/dL]), plasma/serum creatinine (PCr [mg/dL]), and urine flow rate (V [mL/min]) according to the formula:  where  Creatinine clearance is often “corrected” for body surface area (BSA [m2]) according to the formula:  | Increased in: High cardiac output, exercise, acromegaly, diabetes mellitus (early stage), infections, hypothyroidism. Decreased in: Acute or chronic renal failure, decreased renal blood flow (shock, hemorrhage, dehydration, CHF). Drugs: nephrotoxic drugs. | Serum Cr may, in practice, be a more reliable indicator of renal function than 24-hour CiCr unless urine collection is carefully monitored. An 8-hour collection provides results similar to those obtained with a 24-hour collection. ClCr will overestimate glomerular filtration rate to the extent that Cr is secreted by the renal tubules (eg, in cirrhosis). ClCr can be estimated from the serum creatinine using the following formula: 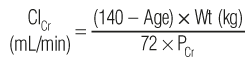 Serial decline in ClCr is the most reliable indicator of progressive renal dysfunction. Also see GFR, estimated (eGFR). Fliser D. Assessment of renal function in elderly patients. Curr Opin Nephrol Hypertens 2008;17:604. [PubMed: 18941354] Stevens LA et al. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 2009;20:2305. [PubMed: 19833901] White CA et al. Performance of creatinine-based estimates of GFR in kidney transplant recipients: a systematic review. Am J Kidney Dis 2008;51:1005. [PubMed: 18455847] |
Cryoglobulins
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Cryoglobulins, serum Negative SST $ Patients should be fasting and blood sample must be drawn in a pre-warmed vacutainer tube, kept at 37°C and immediately transported to the laboratory. | Cryoglobulins are immunoglobulins (IgG, IgM, IgA, or light chains) that precipitate on exposure to the cold. The sample is stored at 4°C and examined daily for the presence or absence of cryoglobulins over a period of 3–5 days. Type I cryoglobulins (25%) are monoclonal immunoglobulins, most commonly IgM, occasionally IgG, and rarely IgA or Bence Jones protein. Type II (25%) are mixed cryoglobulins with a monoclonal component (usually IgM but occasionally IgG or IgA) that complexes with polyclonal normal IgG in the cryoprecipitate. Type III (50%) are mixed polyclonal cryoglobulins (IgM and IgG). | Positive in: Immunoproliferative disorders (multiple myeloma, Waldenström macroglobulinemia, chronic lymphocytic leukemia, lymphoma), collagen vascular disease (SLE, polyarteritis nodosa, rheumatoid arthritis, Sjögren syndrome), hemolytic anemia, infections (eg, HCV, HIV), glomerulonephritis, chronic liver disease. The term “essential mixed cryoglobulinemia” (a vasculitic syndrome) is used to refer to patients with no primary disease other than Sjögren syndrome; other cases are classified as secondary mixed cryoglobulinemia. | All types of cryoglobulins may cause cold-induced symptoms, including Raynaud phenomenon, vascular purpura, and urticaria. Patients with type I cryoglobulinemia usually suffer from underlying disease (eg, multiple myeloma). Patients with type II and III cryoglobulinemia often have immune complex disease, with vascular purpura, bleeding tendencies, arthritis, and nephritis. Typing of cryoglobulins by electrophoresis is not necessary for diagnosis or clinical management. About 50% of essential mixed cryoglobulinemia patients have evidence of hepatitis C infection. Cacoub P et al. Hepatitis C virus infection induced vasculitis. Clin Rev Allergy Immunol 2008;35:30. [PubMed: 18196478] Sargur R et al. Cryoglobulin evaluation: best practice? Ann Clin Biochem 2010;47(Pt 1):8. [PubMed: 20040797] |
Cryptococcal Antigen
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Cryptococcal antigen, serum or CSF Negative SST (serum) or glass or plastic tube (CSF) $$ | The capsular polysaccharide of Cryptococcus neoformans potentiates opportunistic infections by the yeast. The cryptococcal antigen test used is often a latex agglutination test. | Increased in: Cryptococcal infection. | False-positive and false-negative results have been reported. False positives due to rheumatoid factor can be reduced by pretreatment of serum using pronase before testing. Sensitivity and specificity of serum cryptococcal antigen titer for cryptococcal meningitis are 91% and 83%, respectively. Ninety-six percent of cryptococcal infections occur in AIDS patients. Sloan D et al. Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource-limited settings. Cochrane Database Syst Rev 2008:CD005647. [PubMed: 18843697] |
C-Telopeptide, Beta-Cross-Linked
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
C-telopeptide, beta-cross-linked (Beta-CTx), serum Adult male: 60–850 pg/mL Adult female: premenopausal 60–650 pg/mL; postmenopausal 104–1010 pg/mL (age- and laboratory-specific). SST, red <SPAN role=presentation tabIndex=0 id=MathJax-Element-11-Frame class=MathJax style="POSITION: relative" data-mathml='‘> | During bone resorption, osteoclasts secrete a mixture of proteases that degrade the type I collagen fibrils into fragments including C-terminal telopeptide (CTx). One of the fragments is beta-CTx, which is released into blood and is considered a specific marker for increased bone resorption. | Increased in: Osteoporosis, osteopenia, osteomalacia, rickets, Paget disease, hyperparathyroidism, and hyperthyroidism. | Test aids in the diagnosis of medical conditions associated with increased bone turnover, but cannot replace bone mineral density to diagnose osteoporosis. Test may be useful for monitoring antiresorptive treatment in postmenopausal women treated for osteoporosis and individuals diagnosed with osteopenia. Reduced renal function may lead to reduced urinary excretion of beta-CTx and consequent increase in the serum beta-CTx concentration. Also see N-telopeptide, cross-linked. Civitelli R et al. Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporos Int 2009;20:843. [PubMed: 19190842] Delmas PD et al. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int 2000;11:S2. [PubMed: 11193237] Garnero P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther 2008;12:157. [PubMed: 18510379] |
Cyclic Citrullinated Protein Antibody
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Cyclic citrullinated protein antibody (anti-CCP), serum Negative SST, red <SPAN role=presentation tabIndex=0 id=MathJax-Element-12-Frame class=MathJax style="POSITION: relative" data-mathml='‘> | Post-translational deamination of arginine residues by peptidyl arginine deaminase (citrullination) during inflammation results in production of antigenic epitope. Antibodies to citrullinated proteins (particularly filaggrin) are frequently elevated in rheumatoid arthritis (RA). | Increased in: RA (sensitivity 70–80%). | Specificity of anti-CCP (90–95%) for RA is higher than that of rheumatoid factor. Pincus T et al. Laboratory tests to assess patients with rheumatoid arthritis: advantages and limitations. Rheum Dis Clin North Am 2009;35:731. [PubMed: 19962617] Raptopoulou A et al. Anti-citrulline antibodies in the diagnosis and prognosis of rheumatoid arthritis: evolving concepts. Crit Rev Clin Lab Sci 2007;44:339. [PubMed: 17558653] Whiting PF et al. Systematic review: accuracy of anti-citrullinated peptide antibodies for diagnosing rheumatoid arthritis. Ann Intern Med 2010;152:456. [PubMed: 20368651] |
Cytomegalovirus Antibody
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Cytomegalovirus antibody, serum (CMV) Negative SST $$$ | Detects the presence of antibody to CMV, either IgG or IgM. CMV infection is usually acquired during childhood or early adulthood. By age 20–40 years, 40–90% of the population has CMV antibodies. | Increased in: Previous or active CMV infection. False-positive CMV IgM tests occur when rheumatoid factor or infectious mononucleosis is present. | Serial specimens exhibiting a greater than fourfold titer rise suggest a recent infection. Active CMV infection must be documented by viral isolation. Useful for screening of potential organ donors and recipients. Universal prophylaxis reduces infection in transplant recipients. Detection of CMV IgM antibody in the serum of a newborn usually indicates congenital infection. Detection of CMV IgG antibody is not diagnostic, because maternal CMV IgG antibody passed via the placenta can persist in newborn’s serum for 6 months. CMV seronegative blood components are more efficacious than leukocyte-reduced blood components in preventing transfusion-acquired CMV infection. Hyde TB et al. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol 2010;20:311. [PubMed: 20645278] Kalil AC et al. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med 2005;143:870. [PubMed: 16365468] |
D-Dimer
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
D-dimer, plasma Negative Blue $$ | D-dimer is one of the terminal fibrin degradation products. The presence of D-dimers indicates that a fibrin clot was formed and subsequently degraded by plasmin. Essentially, D-dimer is elevated whenever the coagulation system has been activated, followed by fibrinolysis. | Increased in: Deep vein thrombosis (DVT), venous thromboembolism (VTE), pulmonary embolism (PE), disseminated intravascular coagulation (DIC), arterial thromboembolism, pregnancy (especially postpartum period), malignancy, surgery, thrombolytic therapy. | D-dimer assay is a very sensitive test for DIC, DVT and VTE or PE. The D-dimer can be measured by a variety of methods; for example, semiquantitative latex agglutination and quantitative high sensitivity immunoassay (eg, ELISA). The newly developed highly sensitive automated D-dimer tests may be used to exclude PE and DVT: a negative test essentially rules out thrombosis, but a positive test does not confirm the diagnosis, and further testing (eg, ultrasound, CT angiography) is recommended. See Figure 9–23 and Table 8–20 for its use in pulmonary embolism evaluation. Adam SS et al. D-dimer antigen: current concepts and future prospects. Blood 2009;113:2878. [PubMed: 19008457] Galioto NJ et al. Recurrent venous thromboembolism. Am Fam Physician 2011;83:293. [PubMed: 21302870] Green D. Interpreting coagulation assays. Blood Coagul Fibrinolysis 2010;21(Suppl 1):S3. [PubMed: 20855988] |
Dehydroepiandrosterone Sulfate
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Dehydroepiandrosterone sulfate (DHEA-S), serum or plasma Male: 40–500 mcg/dL Female: 20–320 mcg/dL SST, PPT, green, lavender/pink <SPAN role=presentation tabIndex=0 id=MathJax-Element-13-Frame class=MathJax style="POSITION: relative" data-mathml='‘> | DHEA is a 19-carbon endogenous steroid hormone secreted by the adrenal glands. It is converted to DHEA-S in the adrenals, liver, and small intestine. DHEA-S is albumin-bound in the circulation, and there is no diurnal variation in DHEA-S levels. Levels of DHEA-S are about 300 × higher than DHEA and more stable. It serves as the precursor of androgens and estrogens. | Increased in: Adrenal hyperplasia, adrenal cancer, congenital adrenal hyperplasia, polycystic ovarian syndrome. Decreased in: Adrenal insufficiency, hypopituitarism, rheumatoid arthritis (females), insulin, corticosteroids. | DHEAS measurement is typically used along with other steroid and peptide hormones to evaluate adrenal function, to help diagnose adrenal cortex tumors, and polycystic ovarian syndrome (in females). Orally ingested DHEA is converted to DHEA-S when passing through intestines and liver. People taking DHEA supplements have elevated blood levels of DHEA-S. Use by athletes is prohibited by the World Anti-doping Agency. Imrich R et al. Hypothalamic-pituitary-adrenal axis in rheumatoid arthritis. Rheum Dis Clin North Am 2010;36:721. [PubMed: 21092849] Pugeat M et al. Recommendations for investigation of hyperandrogenism. Ann Endocrinol (Paris) 2010;71:2. [PubMed: 20096825] Yildiz BO et al. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord 2007;8:331. [PubMed: 17932770] |
Dexamethasone Suppression Test (Low Dose)
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Dexamethasone suppression test (low dose, overnight), serum or plasma 8:00 AM serum cortisol level: <5 mcg/dL [<140 nmol/L] SST, PPT, green $$ Give 1 mg dexamethasone at 11:00 PM. At 8:00 AM, draw serum/plasma cortisol level. | In normal patients, dexamethasone suppresses the 8:00 AM serum cortisol level below 5 mcg/dL. Patients with Cushing syndrome have 8:00 AM levels >10 mcg/dL (>276 nmol/L). | Positive in: Cushing syndrome (sensitivity is high in severe cases but less so in mild ones; specificity is 70–90% in patients with chronic illness or hospitalized patients). | Good screening test for Cushing syndrome. If this test is abnormal, use high-dose dexamethasone suppression test to determine etiology. (See also Cushing syndrome algorithm, Figure 9–8.) Patients taking phenytoin may fail to suppress because of enhanced dexamethasone metabolism. Depressed patients may also fail to suppress morning cortisol level. Boscaro M et al. Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab 2009;94:3121. [PubMed: 19734443] Elamin MB et al. Accuracy of diagnostic tests for Cushing’s syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab 2008;93:1553. [PubMed: 18334594] Nieman LK et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008;93:1526. [PubMed: 18334580] |
Dexamethasone Suppression Test (High Dose)
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Dexamethasone suppression test (high-dose, overnight), serum or plasma 8:00 AM serum cortisol level: <5 mcg/dL [<140 nmol/L] SST, PPT, green $$ Give 8 mg dexamethasone dose at 11:00 PM. At 8:00 AM, draw cortisol level. | Suppression of plasma cortisol levels to <50% of baseline with dexamethasone indicates Cushing disease (pituitary-dependent ACTH hypersecretion) and differentiates this from adrenal and ectopic Cushing syndrome (see Cushing syndrome algorithm, Figure 9–8). | Positive in: Cushing disease (88–92% sensitivity; specificity 57–100%). | Test indicated only after a positive low-dose dexamethasone suppression test. Sensitivity and specificity depend on sampling time and diagnostic criteria. Measurement of urinary 17-hydroxycorticosteroids has been replaced in this test by measurement of serum cortisol. Bilateral sampling of the inferior petrosal sinuses for ACTH after administration of corticotrophin-releasing hormone has been used to identify the site of adenoma before surgery. Bertagna X et al. Cushing’s disease. Best Pract Res Clin Endocrinol Metab 2009;23:607. [PubMed: 19945026] Boscaro M et al. Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab 2009;94:3121. [PubMed: 19734443] Pecori Giraldi F. Recent challenges in the diagnosis of Cushing’s syndrome. Horm Res 2009;71(Suppl 1):123. [PubMed: 19153521] |
Double-Stranded DNA Antibody
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Double-stranded-DNA antibody (ds-DNA Ab), serum <1:10 titer SST $$ | IgG or IgM antibodies directed against host double-stranded DNA. | Increased in: Systemic lupus erythematosus (SLE; 60–70% sensitivity, 95% specificity) based on >1:10 titer. Not increased in: Drug-induced lupus. | Double-stranded DNA antibodies can be screened by an enzyme-linked immunosorbent assay (ELISA) assay, and, if positive, immunofluorescent antibody testing (IFA) is then performed. High titers are seen only in SLE. Titers of ds-DNA antibody correlate moderately well with occurrence of glomerulonephritis and renal disease activity. (See also Autoantibodies, Table 8–6.) Breda L et al. Laboratory tests in the diagnosis and follow-up of pediatric rheumatic diseases: an update. Semin Arthritis Rheum 2010;40:53. [PubMed: 19246077] Munoz LE et al. Predictive value of anti-dsDNA autoantibodies: importance of the assay. Autoimmun Rev 2008;7:594. [PubMed: 18603024] |
Drug Abuse Screen, Urine
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Drug abuse screen, urine (urine toxicology drug screen, urine drug screen) Negative $ | Testing for drugs of abuse usually involves testing a single urine specimen for a number of drugs, eg, cocaine, opiates, barbiturates, amphetamines, benzodiazepines, cannabinoids, methadone, oxycodone, phencyclidine (PCP), tricyclic antidepressants. Screening tests are often immunoassays, which may not be specific for the tested drug. A positive test may warrant further confirmatory test by gas chromatography-mass spectrometry (GC/MS), the most widely accepted method of drug confirmation. Interpretation of results must take into account that urine concentrations can vary extensively with fluid intake and other biological variables. Adulteration of a urine specimen may also cause erroneous results. | Positive in: Chronic and casual drug users (sensitivity and specificity are assay-dependent). | It is important to know which drugs are included in the drug abuse screen and to understand that the test is qualitative and not quantitative. A single urine drug test detects only fairly recent drug use, and does not differentiate casual use from chronic drug use. The latter requires sequential drug testing and clinical evaluation. Urine drug testing does not determine the degree of impairment, the dose and frequency of drug taken, or the exact time of drug use. A negative result could be due to rapid metabolism/clearance of the drug, not taking drug as prescribed, or diversion of prescribed drugs to others. Cone EJ et al. Urine toxicology testing in chronic pain management. Postgrad Med 2009;121:91. [PubMed: 19641275] Ropero-Miller JD et al. Handbook of Workplace Drug Testing, 2nd ed. Washington, DC, AACC Press, 2008. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29:436. [PubMed: 20924879] |
Epstein-Barr Virus Antibodies
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Epstein-Barr virus antibodies, serum (EBV Ab) Negative SST $$ | Antiviral capsid antibodies (anti-VCA) (IgM) often reach their peak at clinical presentation and last up to 3 months; anti-VCA IgG antibodies last for life. Early antigen antibodies (anti-EA) are next to develop, are most often positive at 1 month after presentation, typically last for 2–3 months, and may last up to 6 months in low titers. Anti-EA may also be found in some patients with Hodgkin disease, chronic lymphocytic leukemia, and some other malignancies. Anti-EB nuclear antigen (anti-EBNA) antibody begins to appear in a minority of patients in the third or fourth week but is uniformly present by 6 months. | Increased in: EB virus infection, infectious mononucleosis. Antibodies to the diffuse (D) form of antigen (detected in the cytoplasm and nucleus of infected cells) are greatly elevated in nasopharyngeal carcinoma. Antibodies to the restricted (R) form of antigen (detected only in the cytoplasm of infected cells) are greatly elevated in Burkitt lymphoma. | Most useful in diagnosing infectious mononucleosis in patients who have the clinical and hematologic criteria for the disease but who fail to develop the heterophile agglutinins (10%) (see Heterophile antibody). EBV antibodies cannot be used to diagnose “chronic” mononucleosis. Chronic fatigue syndrome is not caused by EBV. The best indicator of primary infection is a positive anti-VCA IgM (check for false positives caused by rheumatoid factor). Gulley ML et al. Laboratory assays for Epstein-Barr virus-related disease. J Mol Diagn 2008;10:279. [PubMed: 18556771] |
Erythrocyte Sedimentation Rate
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Erythrocyte sedimentation rate, whole blood (ESR) Males: <10 mm/h Females: <15 mm/h (laboratory-specific) Lavender $ Test must be run within 2 hours after sample collection. | In plasma, erythrocytes (red blood cells [RBCs]) usually settle slowly. However, if they aggregate for any reason (usually because of plasma proteins called acute-phase reactants, eg, fibrinogen), they settle rapidly. Sedimentation of RBCs occurs because their density is greater than plasma. ESR measures the distance in millimeters that erythrocytes fall during 1 hour. | Increased in: Infections (osteomyelitis, pelvic inflammatory disease [75%]), inflammatory disease (temporal arteritis, polymyalgia rheumatica, rheumatic fever), malignant neoplasms, paraproteinemias, anemia, pregnancy, chronic renal failure, GI disease (ulcerative colitis, regional ileitis). For endocarditis, sensitivity is approximately 93%. Decreased in: Polycythemia, sickle cell anemia, spherocytosis, anisocytosis, poikilocytosis, hypofibrinogenemia, hypogammaglobulinemia, congestive heart failure, microcytosis, certain drugs (eg, high-dose corticosteroids). | There is often good correlation between ESR and C-reactive protein (CRP), but discordance between ESR and CRP has been noted in certain inflammatory disorders. Test is typically indicated for diagnosis and monitoring of temporal arteritis, systemic vasculitis and polymyalgia rheumatica. The test is not sensitive or specific for other conditions, although an extremely elevated ESR (eg, >100 mm/h) is useful in developing a rheumatic disease differential diagnosis. The ESR is higher in women, blacks, and older persons. A low value is of no diagnostic significance. The ESR should not be used to screen asymptomatic persons for disease because of its low sensitivity and specificity. Kale N et al. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol 2010;21:417. [PubMed: 20811283] Keenan RT et al. Erythrocyte sedimentation rate and C-reactive protein levels are poorly coordinated with clinical measures of disease activity in rheumatoid arthritis, systemic lupus erythematosus and osteoarthritis patients. Clin Exp Rheumatol 2008;26:814. [PubMed: 19032813] Rosa Neto NS et al. Screening tests for inflammatory activity: applications in rheumatology. Mod Rheumatol 2009;19:469. [PubMed: 19697096] Wu AH et al. Antiquated tests within the clinical pathology laboratory. Am J Manag Care 2010;16:e220. [PubMed: 21250398] |
Erythropoietin
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Erythropoietin, serum or plasma (EPO) 5–30 mIU/mL [5–30 IU/L] SST, PPT $$$ | Erythropoietin (EPO) is a glycoprotein hormone produced in the kidney (peritubular capillary endothelial cells) that induces RBC production by stimulating proliferation, differentiation, and maturation of erythroid precursors. Hypoxia is the usual stimulus for production of EPO. EPO has also been shown to have an important cytoprotective function in the neuronal and cardiovascular systems. | Increased in: Anemias associated with bone marrow hyporesponsiveness (aplastic anemia, iron deficiency anemia), hemolytic anemia, secondary polycythemia (high-altitude hypoxia, COPD, pulmonary fibrosis), EPO-producing tumors (cerebellar hemangioblastomas, pheochromocytomas, renal tumors), kidney transplant rejection, pregnancy, polycystic kidney disease, treatment with recombinant human EPO. Decreased in: Anemia of chronic disease, renal failure, inflammatory states, primary polycythemia (polycythemia vera) (39%), HIV infection with AZT treatment. | EPO levels are useful in differentiating primary from secondary polycythemia and in detecting recurrence of EPO-producing tumors. See diagnostic evaluation of polycythemia (Figure 9–21). Because virtually all patients with severe anemia due to chronic renal failure respond to EPO therapy, pre-therapy EPO levels are not necessary. Patients receiving recombinant human EPO as chronic therapy should have iron studies performed routinely. Landolfi R et al. Polycythemia vera. Intern Emerg Med 2010;5:375. [PubMed: 20237866] Lippi G. Thrombotic complications of erythropoiesis-stimulating agents. Semin Thromb Hemost 2010;36:537. [PubMed: 20632251] Marsden JT. Erythropoietin-measurement and clinical applications. Ann Clin Biochem 2006;43:97. [PubMed: 16536911] |
Estradiol
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Estradiol (E2), serum Adult males: 10–40 pg/mL Adult females:
Adult males: 37–147 pmol/L Adult females:
(*E2 levels vary widely through the menstrual cycle.) SST, red <SPAN role=presentation tabIndex=0 id=MathJax-Element-14-Frame class=MathJax style="POSITION: relative" data-mathml='‘> | In women, estradiol is produced primarily by the granulosa cells of the ovaries by aromatization of androstenedione to estrone, followed by conversion of estrone to estradiol by 17 β-hydroxysteroid dehydrogenase. Smaller amounts of estradiol are also produced by the adrenal cortex and some peripheral tissues (eg, fat cells), and by the testes (in men). E2 is the predominant sex hormone in females. It is responsible for the development of secondary sex characteristics (eg, breast development). E2 levels in premenopausal women fluctuate during the menstrual cycle. They are low at menstruation (<50 pg/mL), rise with follicular development (peak 200–400 pg/mL), drop briefly at ovulation, rise again during the luteal phase, and then drop to menstrual levels. During pregnancy, estrogen levels, including estradiol, rise steadily toward term. | Increased in: Feminization, gynecomastia, precocious puberty, estrogen-producing tumors, hepatic cirrhosis, hyperthyroidism. Decreased in: Primary and secondary hypogonadism. | E2 measurement is of value, together with gonadotropins, in evaluating menstrual and fertility problems in adult females. It is also useful in the evaluation of feminization (including gynecomastia) and estrogen-producing tumors in males. E2 test is used in therapeutic monitoring of human menopausal gonadotropin therapy, estrogen replacement therapy, and antiestrogen therapy (eg, aromatase inhibitor therapy). It is also used for monitoring ovarian hyperstimulation during in vitro fertilization treatment. Johnson RE et al. Gynecomastia: pathophysiology, evaluation, and management. Mayo Clin Proc 2009;84:1010. [PubMed: 19880691] Meczekalski B et al. Hypoestrogenism in young women and its influence on bone mass density. Gynecol Endocrinol 2010;26:652. [PubMed: 20504098] Shulman DI et al. Use of aromatase inhibitors in children and adolescents with disorders of growth and adolescent development. Pediatrics 2008;121:e975. [PubMed: 18381525] |
Ethanol
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Ethanol, serum or plasma (EtOH) 0 mg/dL [mmol/L] SST, red, lavender, PPT $$ Do not use alcohol swab. Do not remove stopper. | Measures serum level of ethyl alcohol (ethanol). | Present in: Ethanol ingestion. | Whole blood alcohol concentrations are about 15% lower than serum concentrations. Each 100 mg/dL of ethanol contributes about 22 mosm/kg to serum osmolality (see Table 8–15). Legal intoxication in many states is defined as >80 mg/dL (>17 mmol/L). Lee H et al. Alcohol-induced blackout. Int J Environ Res Public Health 2009;6:2783. [PubMed: 20049223] Leeman RF et al. Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol 2010;15:109. [PubMed: 20148775] |
Factor Assays
| Test/Range/Collection | Physiologic Basis | Interpretation | Comments |
|---|---|---|---|
Factor assays (coagulation factors II, V, VII, VIII, IX, X, XI, and XII) Blood Blue 50–150% $$$ Deliver immediately to laboratory on ice. Stable for 2 hours. Freeze if assay is delayed > 2 hours. | The partial thrompoplastin time (PTT) and prothrombin time (PT) are the bases for factor assays. Factors VIII, IX, XI, and XII are PTT-based. Factors II, V, VII, and X are PT-based. The factor assay is based on the ability of patient plasma to correct the PTT or PT of specific factor-deficient plasma. Quantitative results are obtained from comparing with a standard curve made from dilutions of normal reference plasma. | Decreased in: Hereditary factor deficiency (eg, hemophilia A, B); acquired factor deficiency secondary to acquired factor-specific inhibitor (eg, factor VIII inhibitor), liver disease (except factor VIII) and DIC (consumptive coagulopathy); vitamin K deficiency or warfarin therapy (II, VII, IX, and X), etc. Severe factor II deficiency may occur in rare patients with lupus anticoagulant. Acquired factor X deficiency may occur in patients with amyloidosis. Patients with von Willebrand disease may have low factor VIII levels. | Although factor assays are typically PTT- or PT-based, chromogenic and immunogenic factor assays are also available for some factors including factors X and VIII. Heparin, hirudin, and argatroban can act as inhibitors and interfere with specific factor assays. Factor assay result needs to be interpreted with caution if non-parrellelism is present (eg, lupus anticoagulant, factor-specific inhibitor). Chromogenic assay is more reliable in this setting. Brenner B et al. Vitamin K-dependent coagulation factors deficiency. Semin Thromb Hemost 2009;35:439. [PubMed: 19598072] Marbet GA. Quantification of coagulation factors and inhibitors. Still a special task. Hamostaseologie 2006;26:38. [PubMed: 16444320] Verbruggen B et al. Improvements in factor VIII inhibitor detection: from Bethesda to Nijmegen. Semin Thromb Hemost 2009;35:752. [PubMed: 20169511] |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


