Kidney, Pediatric: Indications and Utility
SURGICAL/CLINICAL CONSIDERATIONS
Goals of Consultation
Provide preliminary frozen section (FS) differential diagnosis in nephrectomy specimens
Allocate tissue for special studies
Most treatment protocols require fresh and snapfrozen tissue
Evaluate margins
Evaluation of vascular and ureteral margins by frozen section is usually not needed for pediatric renal tumors
Parenchymal resection margin may be evaluated in the case of nephron-sparing surgery
Determine specimen adequacy of biopsy specimens
Change in Patient Management
Differential diagnosis established on FS may influence allocation of tissue for special studies
Diagnosis of bilateral renal tumors on FS biopsies
May influence treatment approach, including renalsparing surgery or nephrectomy
Radiologic correlation is important to rule out nephrogenic rests and avoid unnecessary nephrectomy
Permanent sections preferred for diagnosis
Additional biopsies may be performed if initial biopsy is insufficient for diagnosis
Clinical Setting
Renal tumors are rare in children
Only ˜ 600 cases per year in USA
80% are Wilms tumor
Imaging is used to determine if tumor is resectable
If so, nephrectomy is performed
Biopsy is avoided as rupture increases risk of recurrence and upstages malignant tumors to stage III
If not, biopsy may be performed to confirm histologic diagnosis
Nephrectomy may be performed after preoperative chemotherapy if there is sufficient tumor response
5-10% of tumors are bilateral
Almost all pediatric renal tumors except congenital mesoblastic nephroma are known to occur bilaterally
Bilateral tumors are biopsied to establish diagnosis
Radiologic correlation is important to rule out nephrogenic rests/nephroblastomatosis
Permanent section evaluation is preferable to frozen sections in this clinical scenario
Patients may undergo chemotherapy prior to further surgery
Surgical treatment strategy depends upon response, location, and size of tumors
Unilateral nephrectomy and contralateral renalsparing surgery
Bilateral renal-sparing surgeries
Biopsy may be performed in cases of poor response to chemotherapy of bilateral Wilms tumors
SPECIMEN EVALUATION
Gross
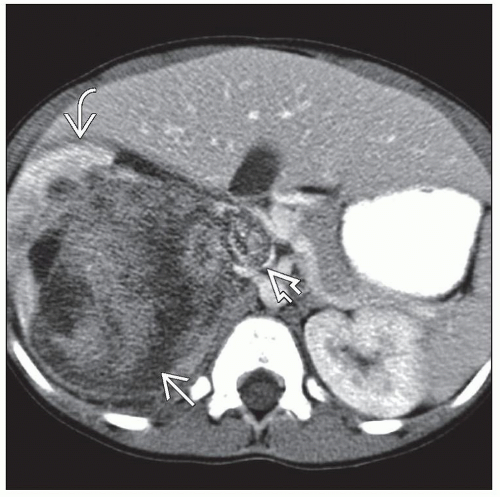
Nephrectomy specimen
Identify structures present
Ureter, renal vessels
Inspect renal vein for tumor thrombus
Vein may retract, resulting in tumor thrombus protruding from lumen
Not considered positive margin if thrombus has not been transected and vascular wall at margin is not invaded
Adrenal may or may not be present
Kidney
Inspect outer surface for any tumor involvement
Important to determine and record whether or not capsule is grossly intact
Photograph intact specimen
Weigh kidney
Weight may serve as eligibility factor in clinical trials
Obtain distal margins of ureter and vessels and place in marked cassette
Ink capsule prior to bisecting kidney
Best 1st cut should pass through midline of kidney in coronal plane
Identify & describe lesions
Location: Hilum, parenchyma
Involvement of renal sinus, renal vein, ureter
Size, number, color, and border
Cysts, necrosis, hemorrhage
Allocate tumors for special studies
Frozen tissue
≥ 1 g snap-frozen in liquid nitrogen or cold isopentane in 2 or more vials
Tumor and nontumor tissue should be frozen
Nephrogenic rests may also be frozen
Electron microscopy
Touch preparations
Flow cytometry
Cytogenetics
Protocol for tissue allocation (same for all pediatric renal tumors)
Protocols of National Wilms Tumor Study Group
Institutional pathology checklist available at www.nwtsg.org is completed when specimens are submitted to this group
Refrigerate specimen in formalin overnight prior to taking permanent sections
Biopsy specimen
Usually 1 or several needle biopsies
Frozen Section
Nephrectomy: Freeze a representative section of tumor
Biopsy: Select 1 needle biopsy to freeze
Cytology
Fine-needle aspiration is not indicated for pediatric renal tumors
Upstages malignant renal tumor to stage III
MOST COMMON DIAGNOSES
Wilms Tumor
Synonymous with nephroblastoma
Most common pediatric renal tumor (˜ 80%)
Peak incidence: 2-4 years of age
Uncommon < 6 months of age
MR appearance
Usually solitary spherical mass that compresses surrounding renal parenchyma
7% multicentric
Well-circumscribed lobulated mass with variegated appearance
Extensive necrosis and hemorrhage are common
May be cystic
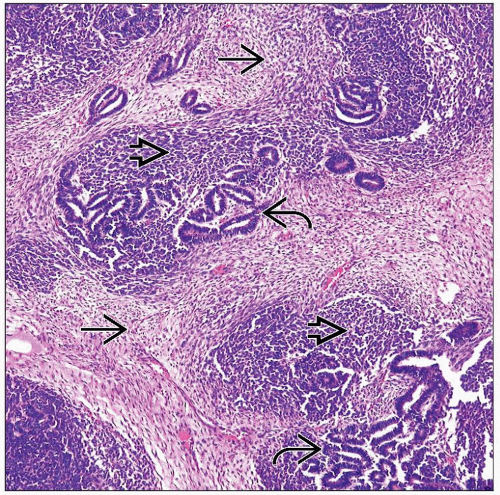
May be triphasic, biphasic, or monophasic
Varying amounts of each component can mimic many other tumors
Differential diagnosis for triphasic Wilms tumor
Nephrogenic rests, nephroblastomatosis
1/3 of kidneys with Wilms tumor also have nephrogenic rests
Differential diagnosis for blastemal-predominant Wilms tumor
Nephrogenic rests, nephroblastomatosis
Renal neuroblastoma
Renal primitive neuroectodermal tumor
Renal lymphoma
Cellular variant of congenital mesoblastic nephroma (CMN)
Differential diagnosis for stromal-predominant Wilms tumor
Classic variant of CMN
Angiomyolipoma
Differential diagnosis for epithelial-predominant Wilms tumor
Metanephric adenoma
Papillary renal cell carcinoma
Differential diagnosis for teratoid Wilms tumor
Renal teratoma, immature
Nephrogenic Rests/Nephroblastomatosis
May be indistinguishable from Wilms tumor histologically if entire rest is not sampled
MR appearance of nephrogenic rests
Oval, oblong, or irregular mass or masses
Perilobular rests usually take shape of renal capsule
Often multiple
MR appearance of diffuse perilobular nephroblastomatosis
Masses that expand cortex, forming cortical rind that preserves cortical shape
Pale masses near cortex
Developmental stages of nephrogenic rests
Dormant/incipient
Sclerosing/regressing
Obsolescent
Hyperplastic
Congenital Mesoblastic Nephroma
Presents congenitally or within 1st year of life
Unilateral tumor arising in central portion of kidney
Classic variant
Composed of intersecting spindle cell fascicles reminiscent of fibromatosis
No consistent translocation identified
Cellular variant
Composed of plump spindle cells
ETV6-NTRK3 gene fusion, t(12;15)(p13;q25)
Identical translocation present in congenital fibrosarcoma
Clear Cell Sarcoma of Kidney
Mean age at diagnosis: 3 years
Wide array of histologic variants
Classic variant
Clear cells arranged within delicate fibrovascular network
Differential diagnosis
Adult-type clear cell renal carcinoma
Classic variant of CMN (may resemble spindle cell variant of clear cell sarcoma of kidney)
Rhabdoid Tumor
Mean age at diagnosis: ˜ 17 months
> 90% diagnosed by 3 years of age
Sheets of large cells
Large round nuclei with prominent nucleoli
Abundant eosinophilic cytoplasm
Cytoplasmic eosinophilic perinuclear inclusions
Immunohistochemistry
SNF5 (INI1/BAF47) negativity is specific for malignant rhabdoid tumors
Positivity for EMA and cytokeratin
Differential diagnosis
Rhabdomyosarcoma (rare in kidney, most commonly develops secondary to Wilms tumor)
Primitive Neuroectodermal Tumor
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree