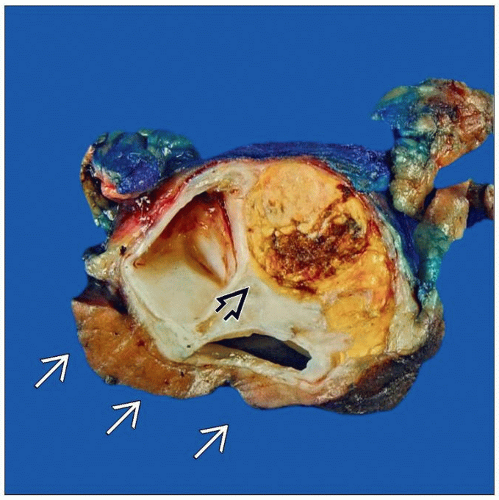
Kidney, Adult: Diagnosis and Margins
 A nephrectomy removes the kidney, ureter, major vessels, and may remove the adrenal. Margins are rarely involved and intraoperative consultation is often not necessary. (Courtesy S. Tickoo, MD.) |
SURGICAL/CLINICAL CONSIDERATIONS
Goal of Consultation
Confirm presumed diagnosis of renal cell carcinoma (RCC) in solid renal lesions or of urothelial carcinoma in renal pelvis lesions
Diagnose cystic renal lesion
Evaluate parenchymal margin when partial nephrectomy is performed
Change in Patient Management
If RCC is confirmed, definite surgery (partial or complete nephrectomy) will be performed
If urothelial carcinoma is confirmed, frozen section of ureter may be performed
Additional bladder cuff margin may be taken
Positive parenchymal margin in partial nephrectomy may result in additional kidney resection or performance of complete nephrectomy
Clinical Setting
Renal masses usually have characteristic findings by imaging
Core needle biopsies or fine-needle aspirations may not be diagnostic
Typically, appropriate management of renal masses is complete surgical excision
Most common clinically evident renal mass is RCC
If mass size requires complete nephrectomy, intraoperative consultation may not be necessary
Smaller asymptomatic masses detected by imaging are more likely to be lesions other than carcinoma
Partial nephrectomy maintains renal function and is preferred in some patients
Indications include
Lesions likely to be benign
Small cancers
Compromised renal function
Single kidneys
Bilateral lesions
Frozen sections may be performed to confirm clinical suspicion of neoplasm
Helpful for lesions with unusual radiographic findings or clinical history
SPECIMEN EVALUATION
Gross
Partial nephrectomy
Consists of a portion of kidney, usually with little surrounding nonrenal tissue
No major vessels or ureter will be present
Cut parenchymal surface is identified
Examine surface for any areas with possible tumor involvement
Ink parenchymal margin
Serially section specimen perpendicular to margin
Identify closest approach of tumor to margin
If tumor is well defined and a rim of normal tissue is present between tumor and margin, gross evaluation is highly predictive of a microscopically negative margin
If gross appearance shows possible tumor involvement, tissue may be taken for frozen section
Tumor bed biopsies
In some centers, biopsies of cut surface of remaining kidney after a partial nephrectomy are performed
Tumor bed biopsies consist of small fragments of tissue and are completely frozen
Radical nephrectomy
Specimen consists of kidney, ureter, renal vein and artery, perinephric fat, and surrounding Gerota fascia
Adrenal gland may or may not be present
Distal margins of ureter and vessels are excised and placed in marked cassettes
Outer aspect of specimen is examined to identify any areas of tumor involvement
Tumor involvement of renal vein is usually identified by preoperative imaging
Tumor may be seen extending from hilum into vein
If tumor is identified at margins, differential inking &/or other identification should be utilized to identify these areas for eventual sampling
Outer portion of specimen is inked
Probe is placed into ureter
Incision is made along probe to bisect ureter and plane of section is extended to bisect kidney
Allows examination of entire urothelium
All lesions are identified including size, number, location, and relationship to margins
Many renal tumors can be identified by their gross features
Tumor identification may not require a frozen section
Frozen Section
Parenchymal margin of partial nephrectomy can be submitted for frozen section if gross lesion is present
In some cases, frozen section of tumor can be helpful for comparison with findings at margin
Tumor bed biopsies of a partial nephrectomy should be entirely frozen
Multiple sections of cystic renal lesions and oncocytic lesions may be needed to confirm diagnosis
Should only be attempted in unusual circumstances in which finding of carcinoma would alter surgical approach
MOST COMMON DIAGNOSES
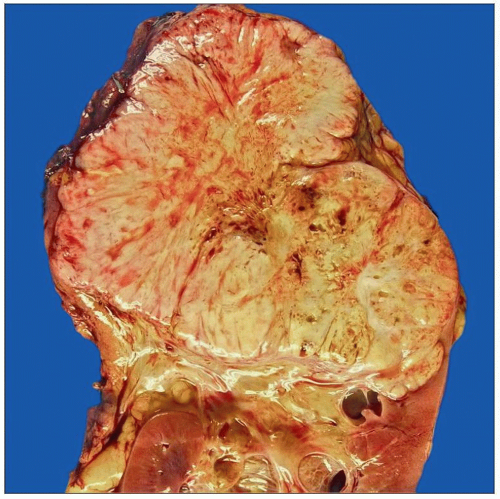
Clear Cell Renal Cell Carcinoma
Most common renal tumor
Circumscribed or lobulated mass or masses with pushing borders
Majority in upper pole
Tumor may bulge into surrounding adipose tissue but invasion outside of kidney is rare
˜ 15% multifocal
Golden yellow to red color
May have areas of hemorrhage, necrosis, and cystic degeneration
Preoperative renal artery embolization can result in extensive infarction and necrosis
Gross appearance is often heterogeneous
Cells have clear to eosinophilic cytoplasm arranged in nests or solid sheets within a delicate fibrovascular network
Blood lakes due to delicate vasculature are typical
Nuclear grade ranges from low to high
RCC with prominent cystic changes may be identified by an inner irregular surface or papillary projection(s)
Can be difficult to diagnose on frozen section
Multilocular cystic RCC consists of only thin-walled cysts without solid areas
May not be possible to distinguish from cystic nephroma intraoperatively
Papillary Renal Cell Carcinoma
Tumors may appear circumscribed with a fibrous pseudocapsule
Characteristic brown color is due to hemosiderin deposition
Tumors can be yellow due to foamy histiocytes
Tumors may have papillary, solid, or trabecular architecture
Papillary architecture gives a soft friable appearance
Can be mistaken grossly for necrosis
Cystic degeneration may occur
True necrosis may be present
Foamy histiocytes are commonly present within the fibrovascular cores
2 types
Type 1 tumors: Cells with scant cytoplasm and lowgrade cytology
Type 2 tumors: Eosinophilia, nuclear pseudostratification, and high-grade cytology
Collecting Duct Renal Cell Carcinoma
Tumors are predominantly located within renal medulla but may extend into renal cortex in larger tumors
Irregular firm to hard gray-white masses
Necrosis is common
High-grade nuclear features
Desmoplasia common
Inflammatory infiltrates common
Some cases closely resemble high-grade urothelial carcinoma
Chromophobe Renal Cell Carcinoma
Well-circumscribed tan to brown mass
May have a central scar, hemorrhage, or necrosis
Most have a solid growth pattern
Nested, alveolar, and other patterns can be present
Nuclear features
Irregular wrinkled nuclei (raisinoid cells)
Prominent perinuclear halos
Binucleated cells
Cytoplasm
Abundant, granular, and pale pink
Eosinophilic variant has darkly staining cytoplasm
Tend to have fewer perinuclear halos and wrinkledappearing nuclei
Well-defined cellular membranes
Clear cells often present
May be at periphery
May be difficult to distinguish from oncocytoma
Frozen section diagnosis of “oncocytic renal neoplasm” may be preferable unless a chromophobe carcinoma can clearly be diagnosed
Oncocytoma
Well-circumscribed tan to dark brown mass
Central scar in 1/2
Helpful feature to support identification of these lesions by imaging
No necrosis
Can resemble chromophobe RCC grossly
Usually, pattern is of nests of cells in a hypocellular stroma
Can also form tubules
Sheets of cells, like chromophobe RCC, is less common
Nuclei
Round and regular
No perinuclear halos
Cytoplasm
Granular and eosinophilic
Cell borders not distinct
Clear cells absent or only focally present
Definitive diagnosis may not be possible on frozen section
Angiomyolipoma
Benign tumor within perivascular epithelioid cell tumor (PEComa) class of tumors
Usually well circumscribed
Imaging findings may be diagnostic due to admixture of adipose tissue
May be confined to kidney or extend through capsule
Rare cases involve renal vein or regional lymph nodes
Consists of blood vessels, smooth muscle, and adipose tissue
Hemorrhage may be present
Blood vessels have thick hyalinized walls
Spindle cells appear to spin off of vascular walls
Amount of each component is variable
Spindle cells may be bland or show focal nuclear atypia
Smooth muscle cells can also be epithelioid in appearance
Diagnosis can be difficult on frozen section
Adipose tissue can be misinterpreted as artifactual clefts on frozen section
Tumors with a predominant smooth muscle component can resemble a smooth muscle neoplasm
Spindle cells can misinterpreted as a malignant spindle cell tumor, such as sarcomatoid RCC
˜ 1/2 of cases are associated with tuberous sclerosis
More likely to be multiple &/or bilateral
Rarely associated with RCC
Urothelial Carcinoma
May occur as a papillary lesion involving renal pelvis or as an infiltrative lesion, which involves renal parenchyma
Histologically similar to urothelial carcinoma of bladder
Carcinomas invasive into renal parenchyma can be difficult to differentiate from collecting duct carcinoma or other centrally located high-grade RCCs
Malakoplakia
Most common in patients with immunosuppression due to organ transplant, chemotherapy for malignancy, or diabetes
Thought to be due to failure of immune system to destroy bacteria
Forms soft yellow plaques and nodules
Usually small (< 1 cm) but can be up to 4 cm
Proliferation of histiocytes with eosinophilic cytoplasm
Termed von Hansemann cells
Michaelis-Gutmann bodies are pathognomonic and can be identified on frozen sections
Concentric targetoid (“owl eye”) basophilic bodies seen in cytoplasm and extracellularly
Thought to be calcified phagosomes
Xanthogranulomatous Pyelonephritis
Associated with urinary tract obstruction
Often associated with renal calculi
Single or multiple golden yellow lesions, typically with hydronephrosis
Usually located in renal pelvis
Rarely in renal capsule or adjacent adipose tissue
Can resemble clear cell RCC grossly
Can appear to invade renal parenchyma
Aggregates of foamy histiocytes are associated with a mixed inflammatory infiltrate
Fine capillaries can be present
Lack the delicate network of fibrovascular septae of clear cell RCC
Nuclei of histiocytes are bland
Cystic Lesions
Renal cysts can be congenital, sporadic, &/or acquired due to long-term hemodialysis
Majority are benign
Hemorrhage &/or inflammatory changes can make distinction from solid neoplasms difficult by imaging
Increased risk of RCC
All cysts should be carefully examined for mural nodules or papillary projections
RCC can have extensive necrosis and appear cystic, with a gross appearance mimicking a hemorrhagic simple cyst
Multiple sections may be required to locate a focus of RCC within cyst wall
Acquired cystic kidney disease occurs in > 30% of patients with end-stage renal disease
Papillary hyperplasia is common in cysts
Adenomas are frequent and often multiple
5-10% of patients develop RCC
50% of carcinomas are multifocal
Many small and found incidentally in transplant nephrectomies
Some metastasize and cause death
Multiple histologic types occur
Extensive sampling may be necessary to diagnose carcinoma
In general, not requested as part of intraoperative consultation
Cystic Nephroma/Mixed Epithelial and Stromal Tumor (MEST)
Rare benign tumors
Most common in premenopausal women
Can resemble multilocular cystic RCC on imaging
Debated whether these tumors are variants of same tumor type or are separate tumor types
Cystic nephroma
Also known as multilocular cystic nephroma
Composed of simple cysts lined by a single cell layer of cuboidal cells
May have a hobnail appearance
No solid areas
Thin fibrous septae may have appearance of ovarian stroma
Mixed epithelial and stromal tumor
Combination of cysts and solid areas
Lining cells can be cuboidal, urothelial, or be ciliated
Stromal component consists of fibrous tissue, smooth muscle, adipose tissue, or has a spindle cell ovarian appearance
Papillary Adenomas
Usually small incidental lesions
May be multiple
Adenomas are < 5 mm in size
Smaller lesions are classified as tubulopapillary hyperplasia
Low-grade nuclei
Papillary or tubular architecture
Do not have cells resembling those of clear cell or chromophobe carcinomas
Not encapsulated
May have an irregular border
Can be associated with foamy macrophages or psammoma bodies
May be difficult to distinguish from low-grade carcinoma when present at a parenchymal margin on frozen section
Usually located away from main mass
Often histologically distinct from main mass
Lymphoma
Usually, well-defined homogeneous gray to white mass involving cortex or medulla
Extensive necrosis may be present, making interpretation of frozen sections difficult
Most common is large B-cell type
Metastases
Often, prior clinical history of malignancy is present
Metastases to RCC have been reported
May be due to the high vascularity of RCC
REPORTING
Gross
If lesion has gross features of RCC, this finding can be reported
Gross confirmation of negative parenchymal margin of partial nephrectomy is highly predictive of no residual carcinoma
Frozen Section
If findings characteristic for a specific neoplasm are present, a diagnosis may be reported
Often, categorization of lesion as benign or malignant provides surgeon with sufficient information
In difficult cases, such as cystic lesions or oncocytic neoplasms, deferment to permanent sections may be necessary
For the parenchymal margin of a partial nephrectomy, presence or absence of tumor at margin should be reported
Likelihood of a positive margin in absence of a gross lesion is < < 5%
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree