|
Multicystic Renal Cell Neoplasm of Low Malignant Potential |
Cystic Clear Cell Renal Cell Carcinoma |
Age |
Peak sixth decade, mean age at diagnosis 54.3 y (30-80 y) |
Adults, peak incidence in sixth decade. Earlier incidence (starting second decade) in familial renal carcinoma syndromes such as vHL syndrome |
Location |
Almost always unifocal |
Usually unifocal. Multifocal and bilateral in <5% of cases, mainly in familial syndromic setting |
Symptoms |
Pain, hematuria yet most often asymptomatic |
Currently, incidentally detected during abdominal and pelvic imaging |
Signs |
Abdominal mass, many incidentally detectable on imaging studies |
Pain, hematuria, flank mass are the classic triad of presentation |
Etiology |
Unknown. vHL gene mutation documented in up to 25% of cases |
Somatic vHL gene inactivation in two-third of sporadic cases. Inherited germ line vHL mutation (3p25-26) in vHL syndrome |
Gross and Histology |
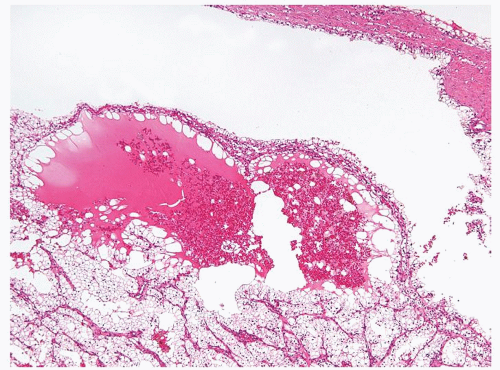
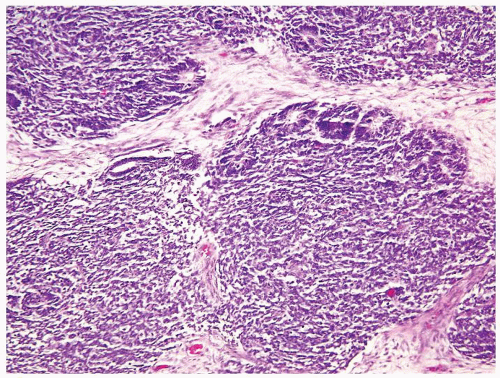
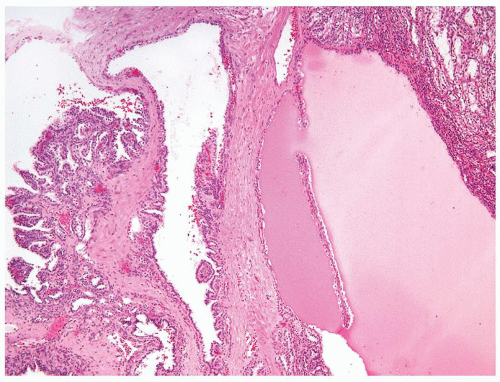
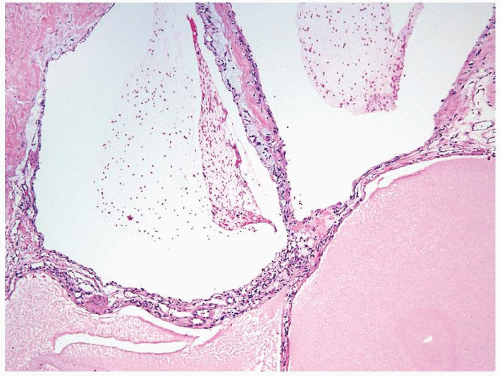
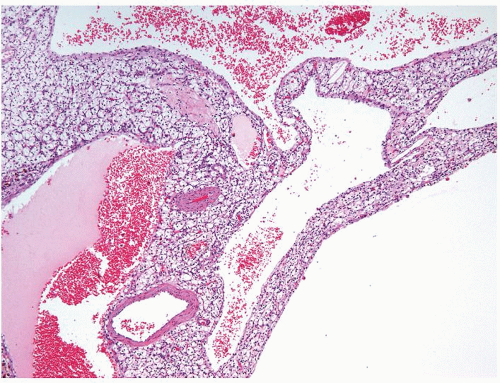
Grossly, multilocular cystic gross appearance, encapsulated, mean size 4.9 cm (1-14 cm)
Cysts have smooth lining filled with straw-colored fluid
Solid component limited to small-sized nonexpansile yellow-gray areas within septa
No necrosis
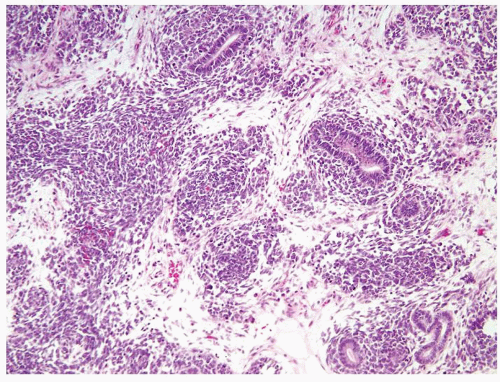
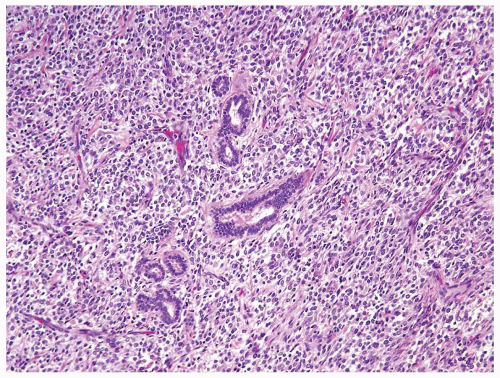
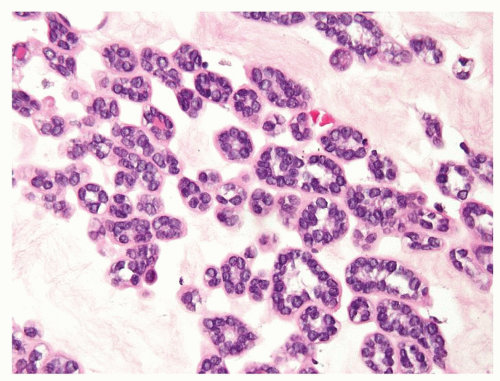
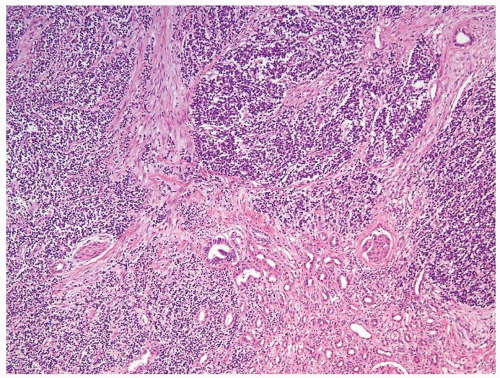
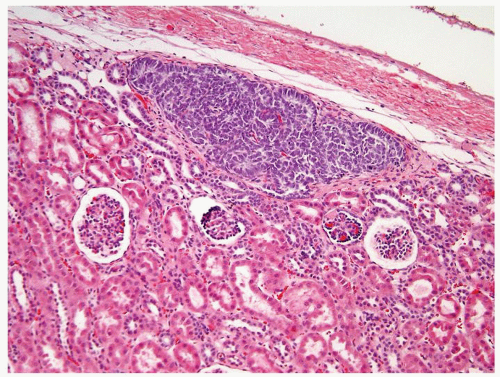
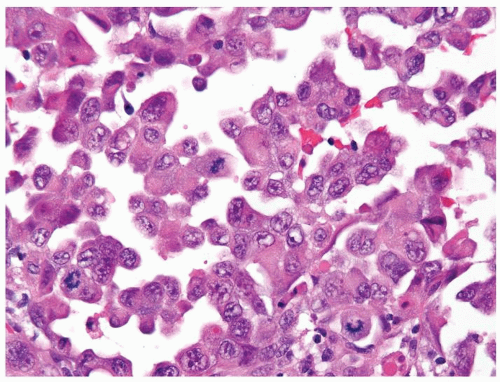
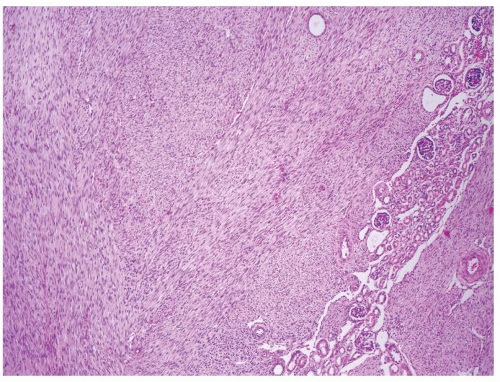
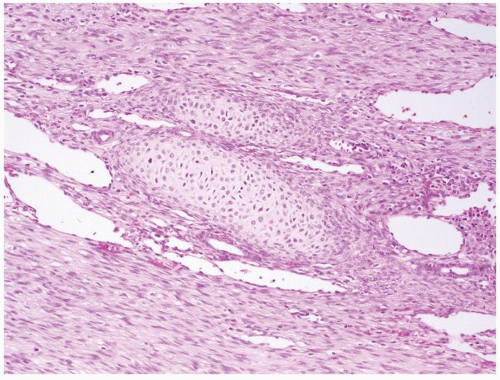
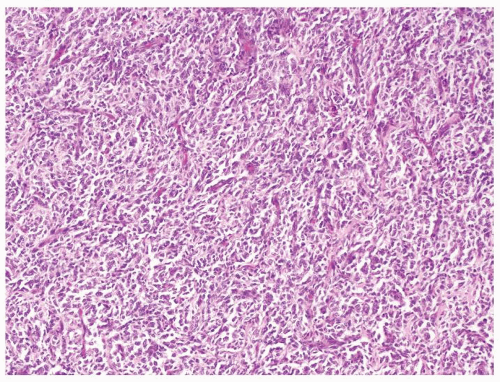
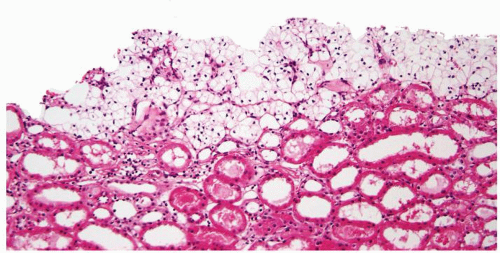
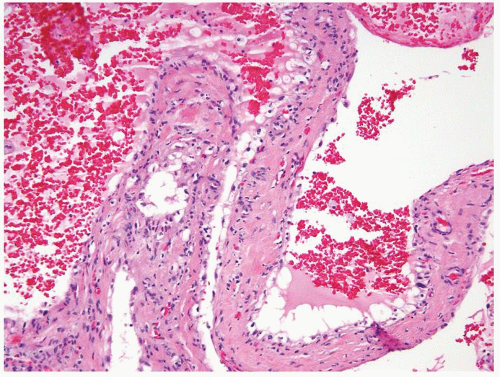
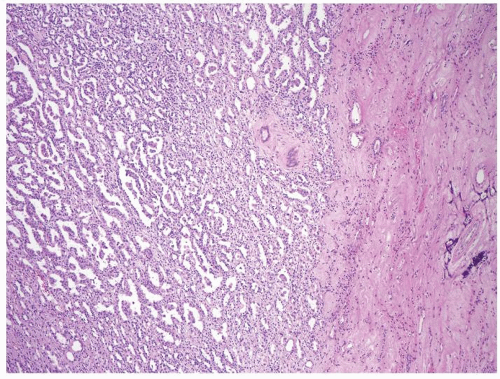
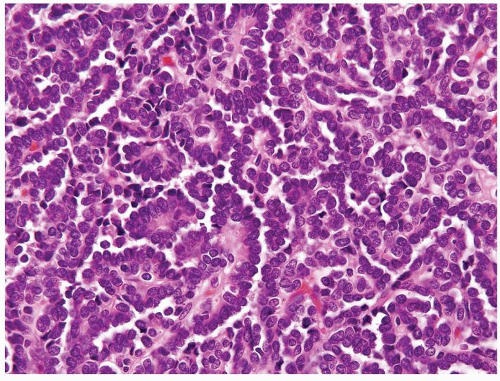
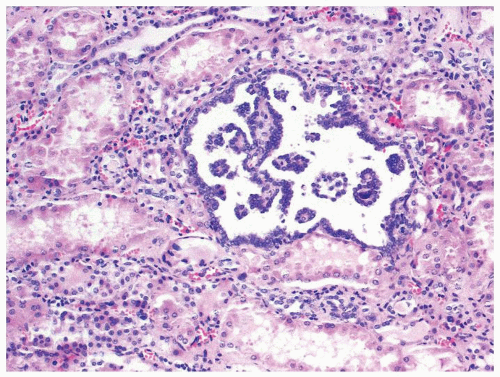
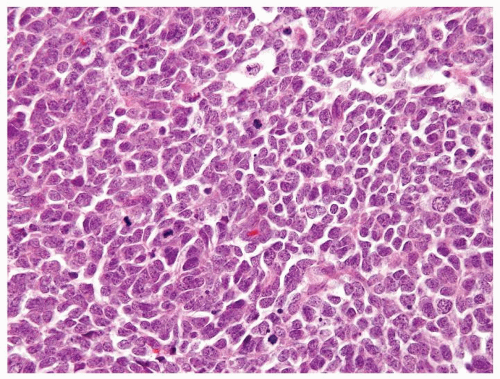
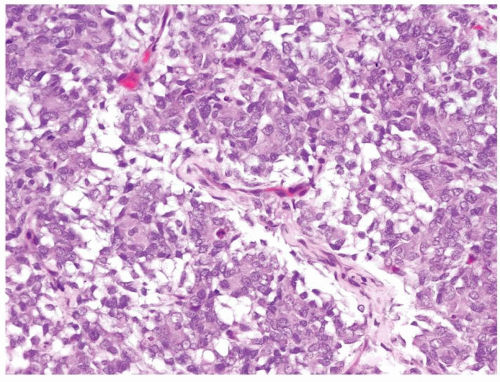
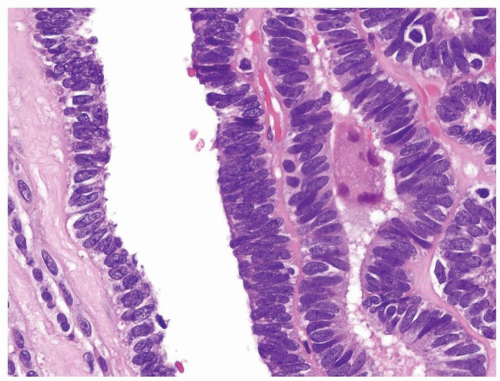
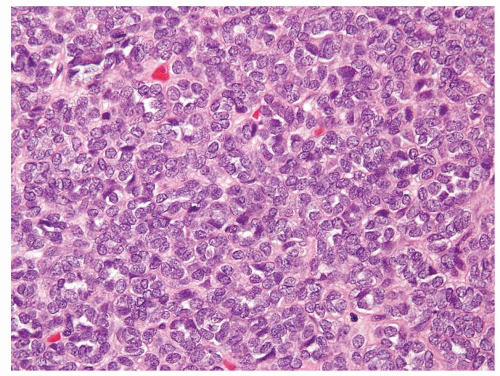
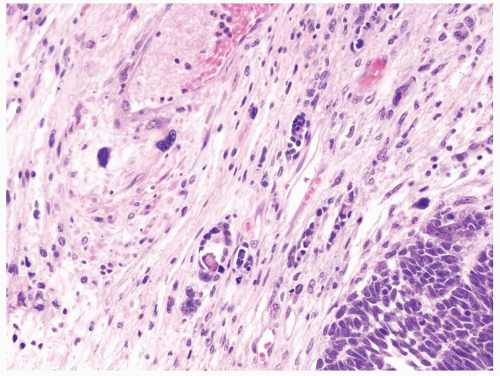
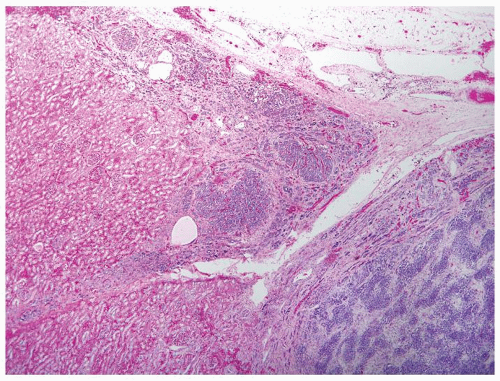
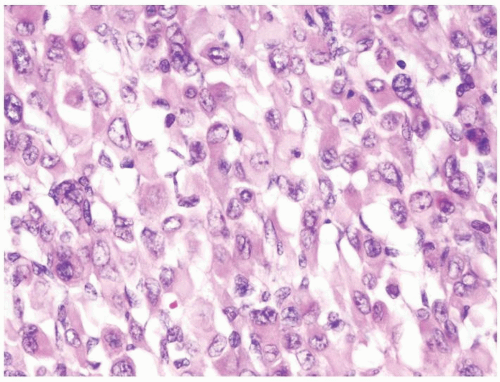
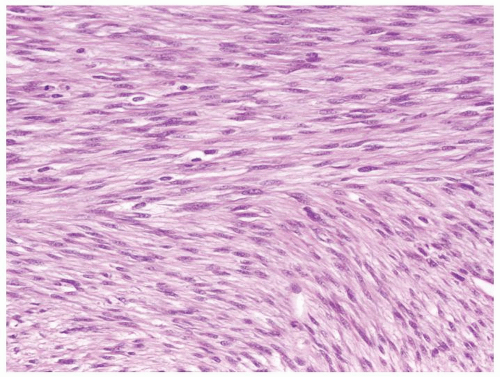
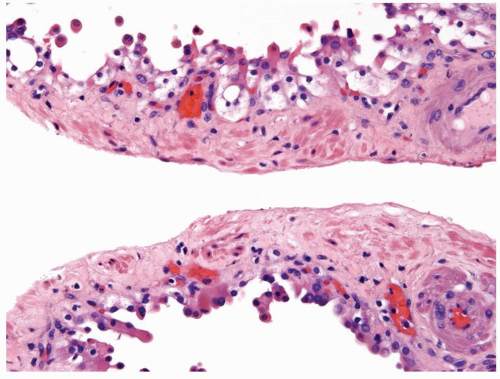
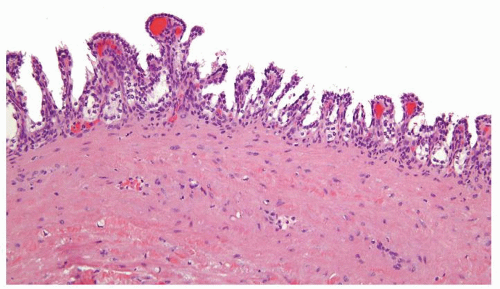
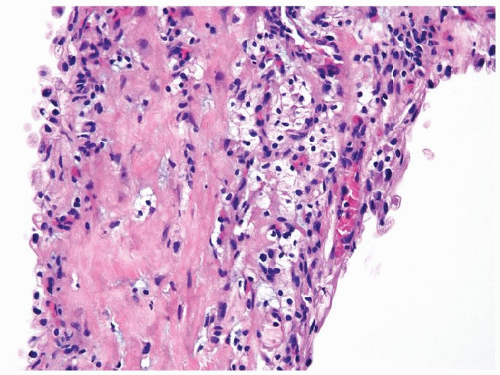
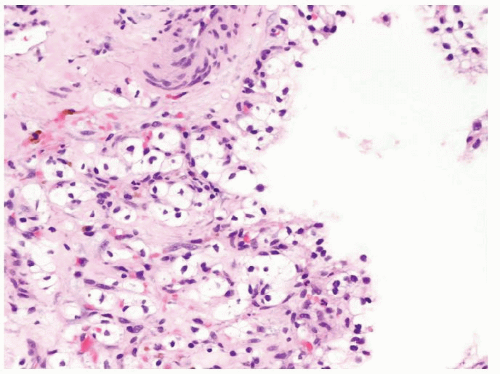
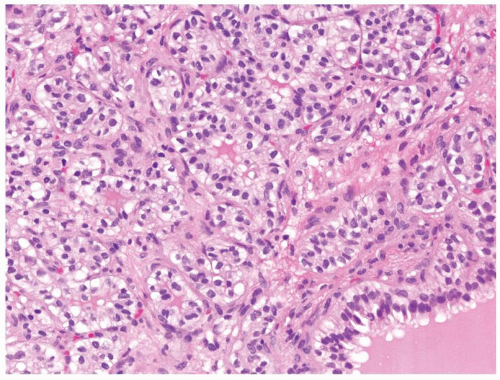
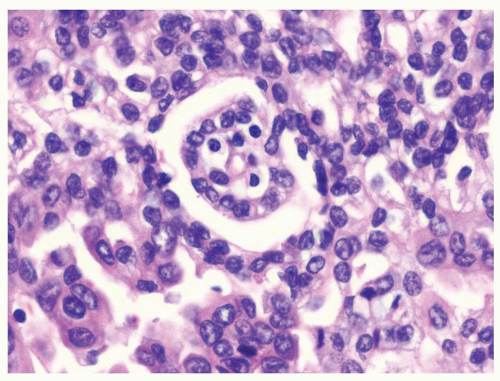
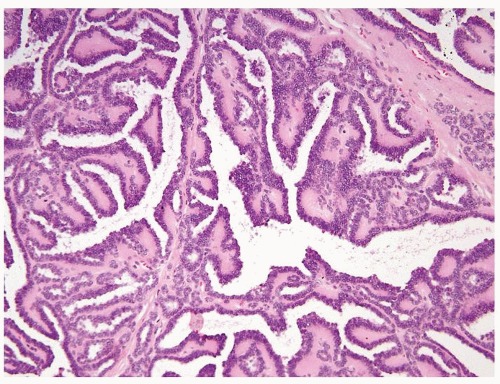
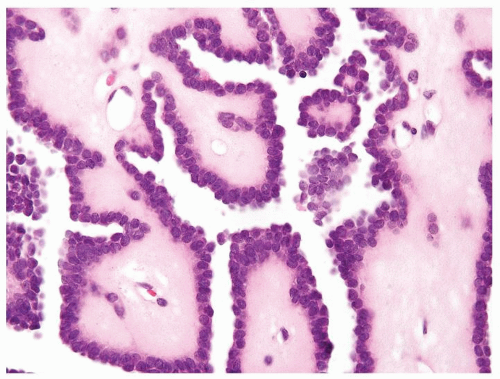
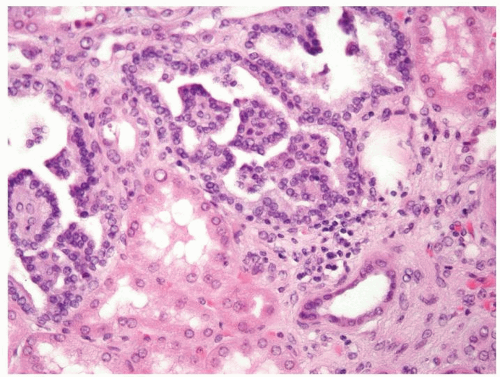
Variable-sized cysts lined by flattened to cuboidal clear cells. Occasional septa contain small aggregates of epithelial cells typical of clear cell RCC (Figs.2.9.1, 2.9.2, 2.9.3, 2.9.4)
Optically clear cytoplasm, distinct cell border, and round to ovoid nuclei
Fuhrman grade 1 or 2
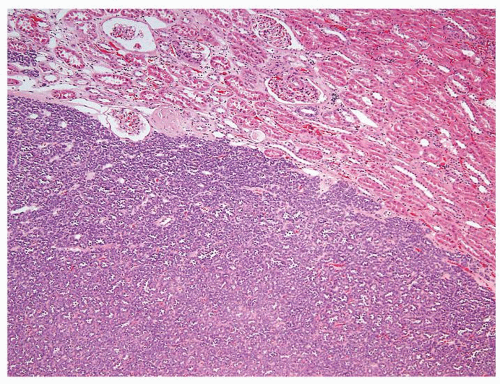
Tumor is organ confined and does not invade vessels
|
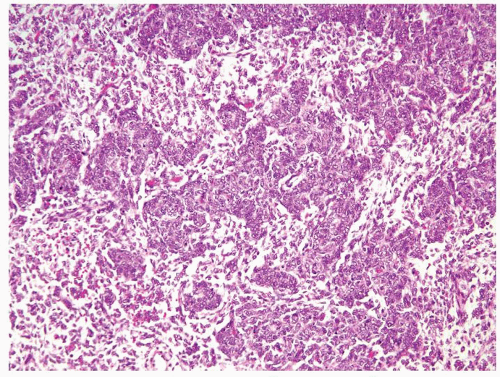
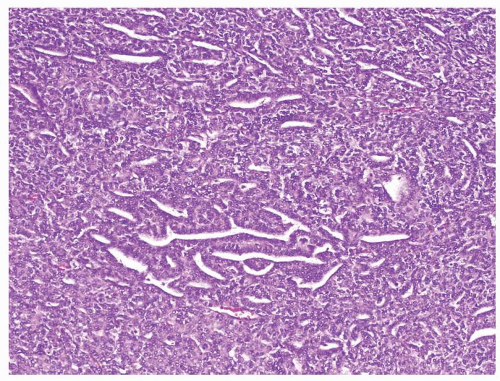
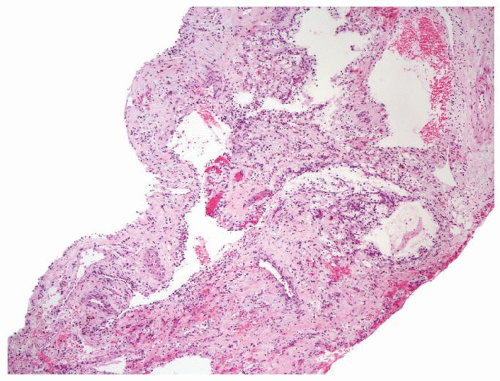
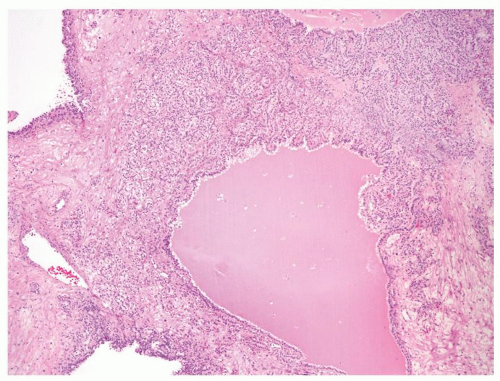
Solid and cystic mass with distinct pushing borders. Mean size 7 cm (wide size range)
Cysts have smooth lining filled with straw-colored fluid
Cystic spaces surrounded by nodular solid areas, typically golden to yellow in color
Associated necrosis and hemorrhage are relatively common in larger masses. Calcifications are encountered
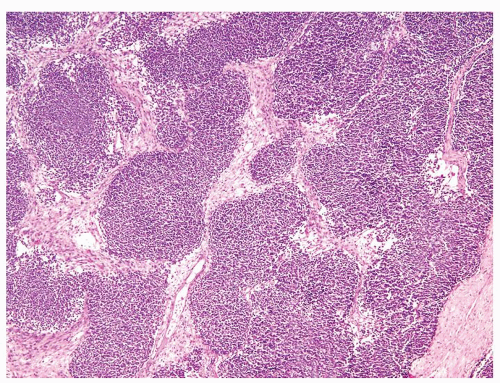
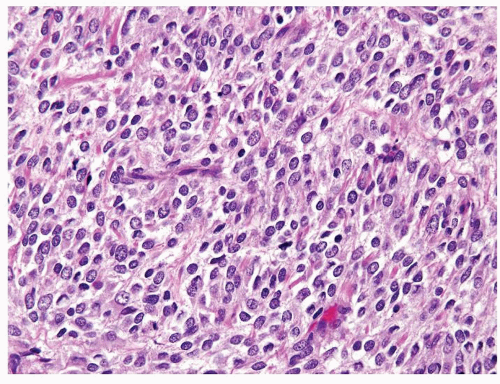
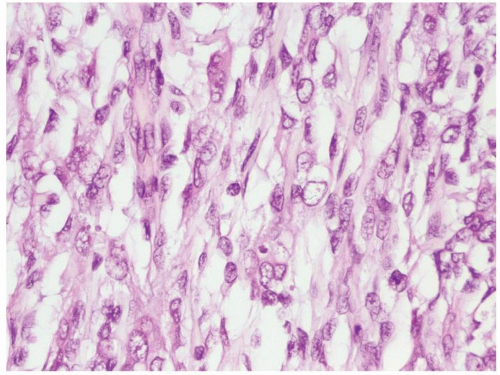
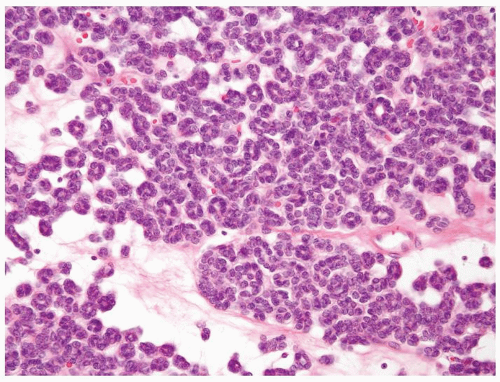
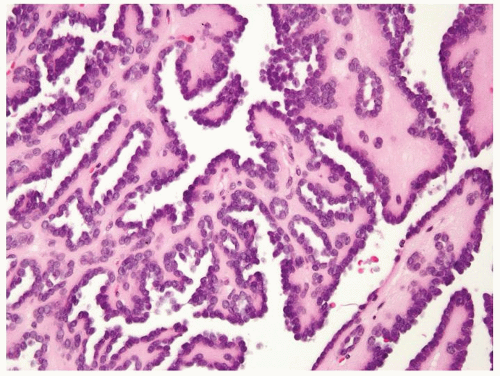
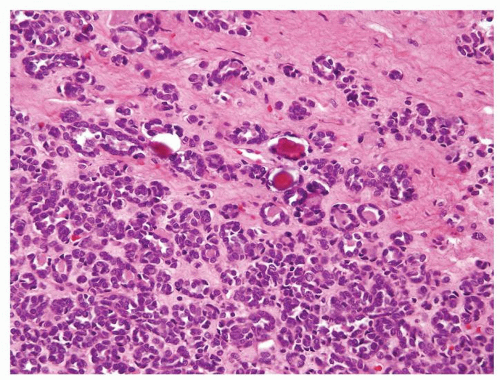
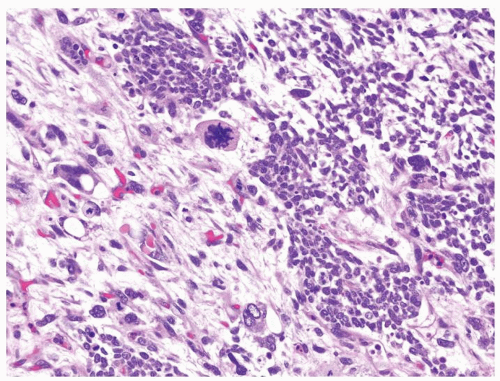
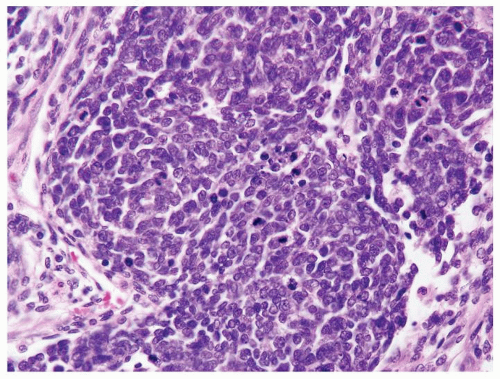
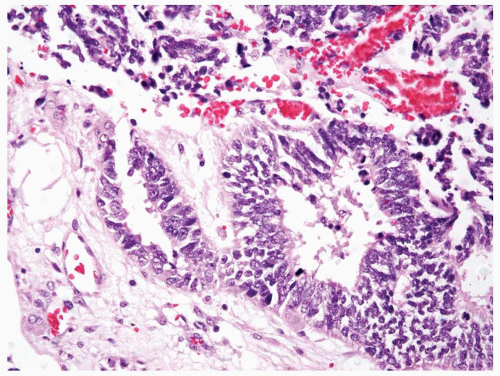
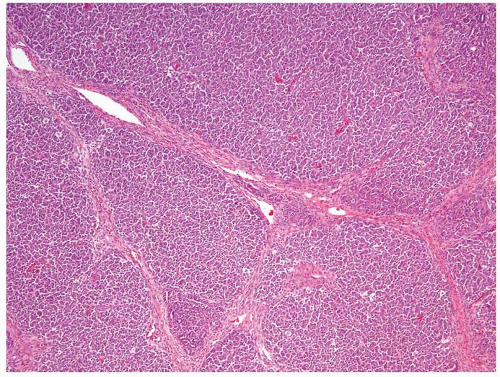
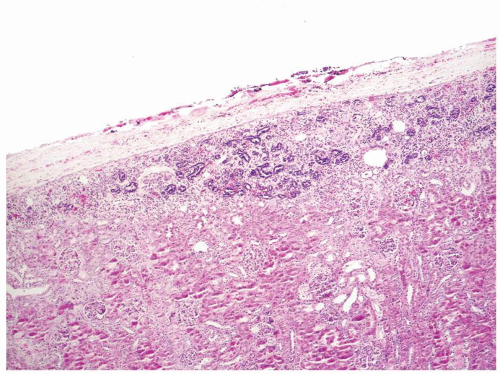
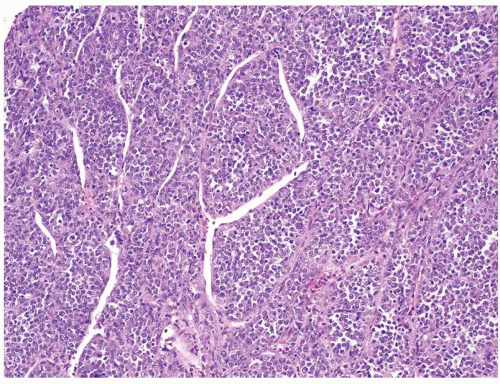
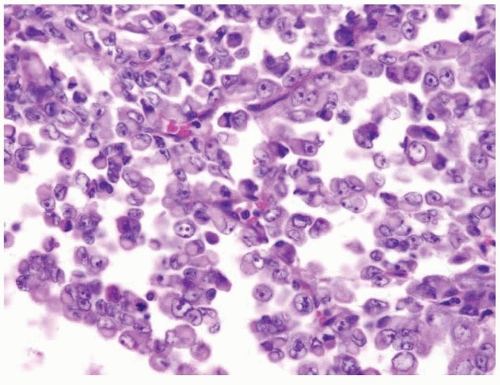
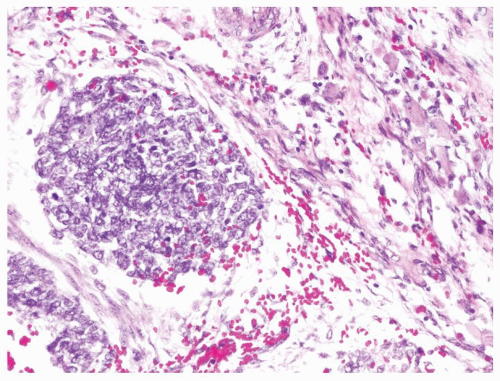
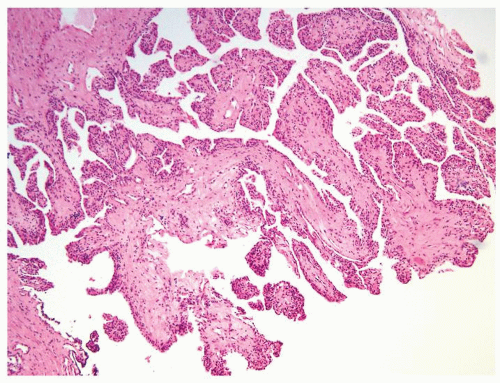
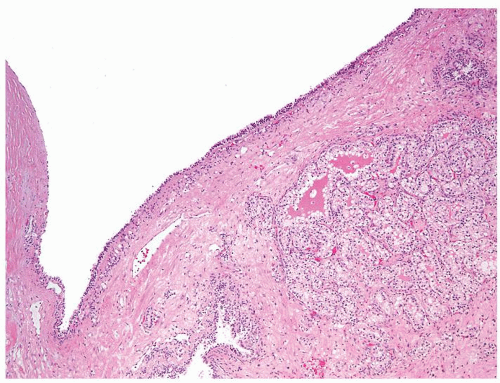
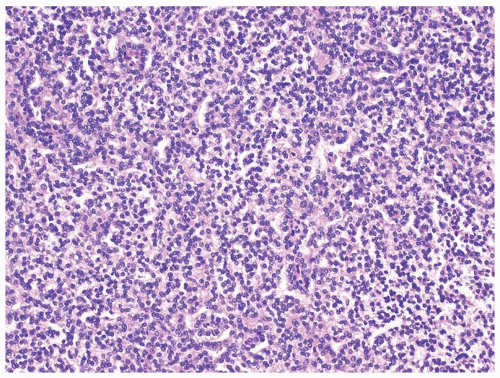
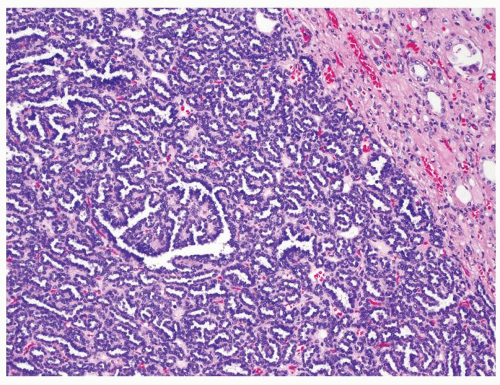
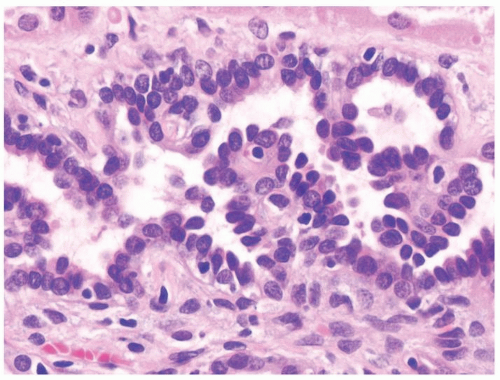
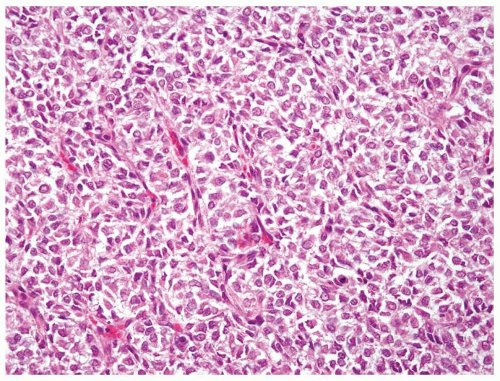
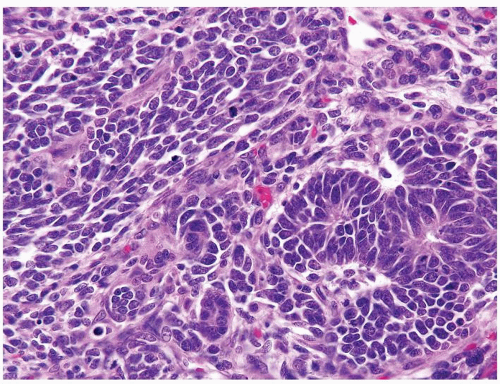
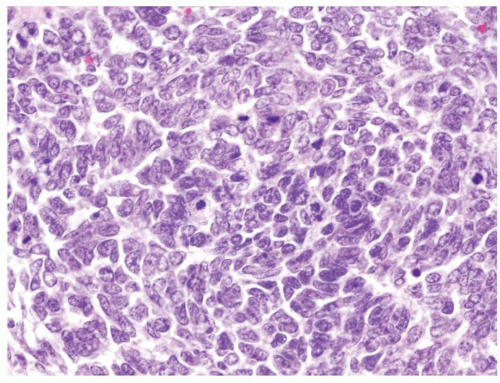
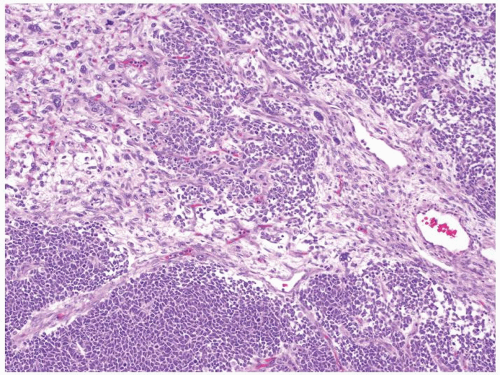
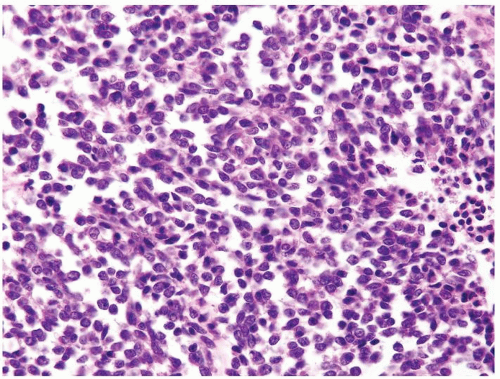
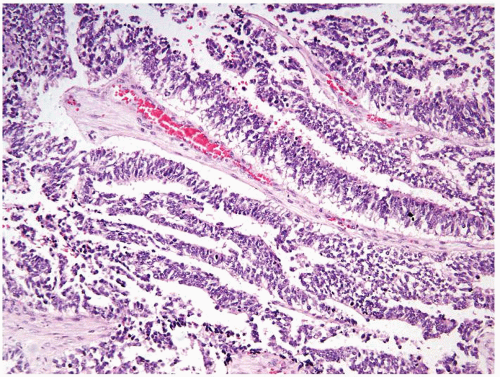
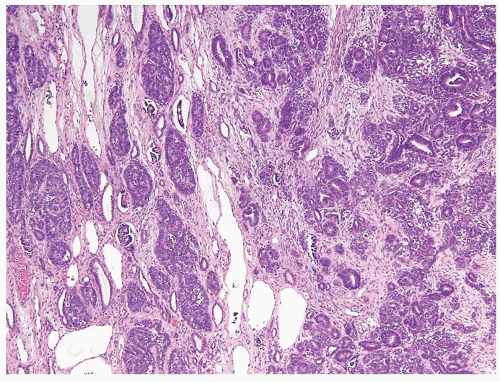
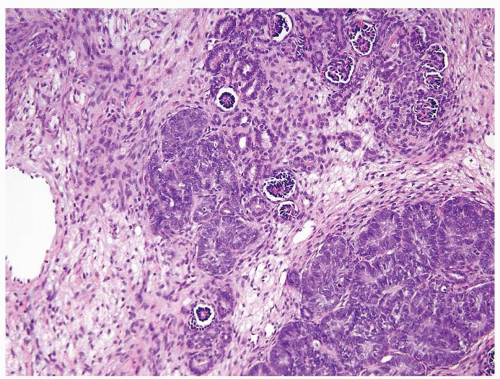
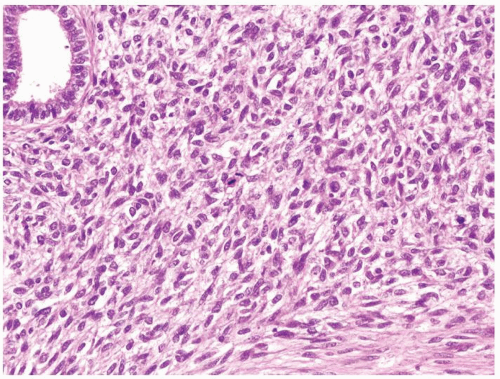
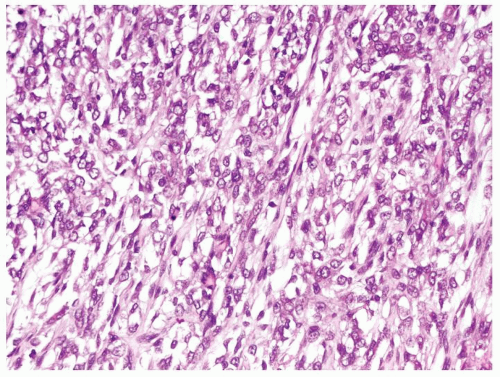
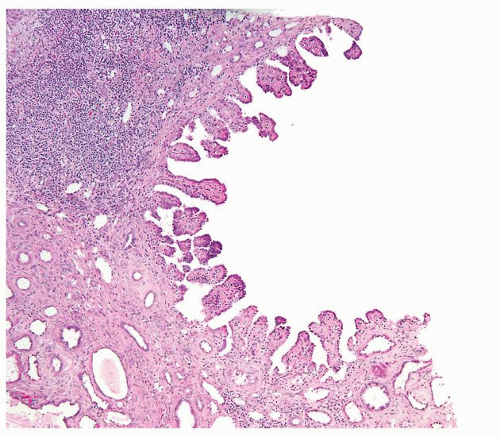
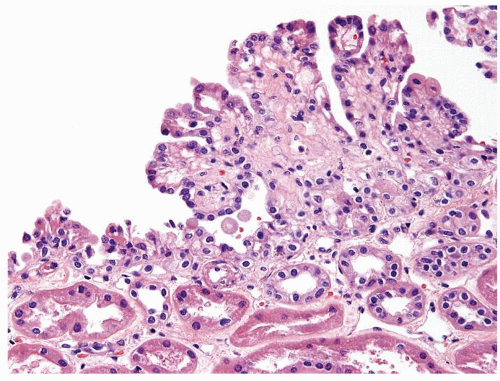
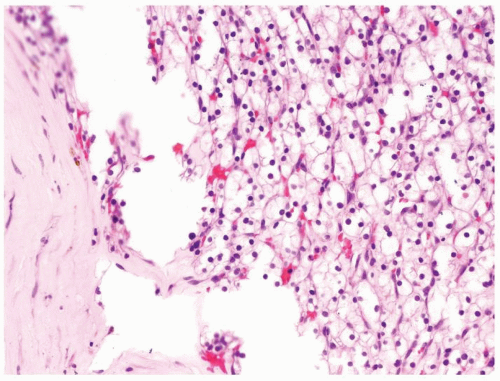
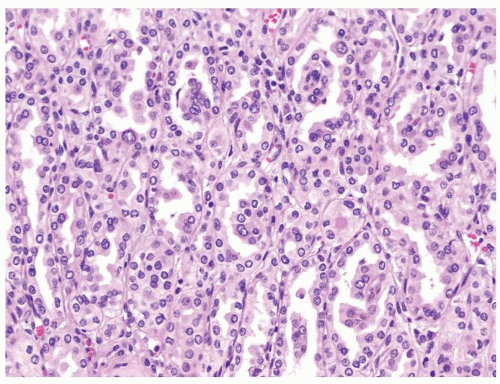
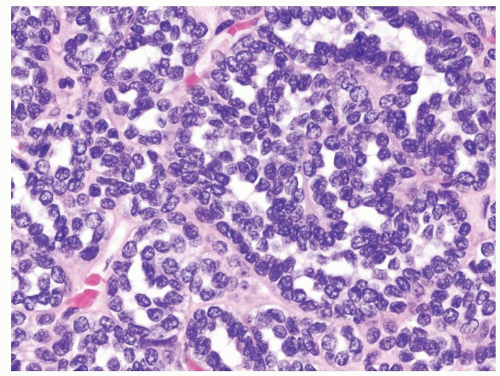
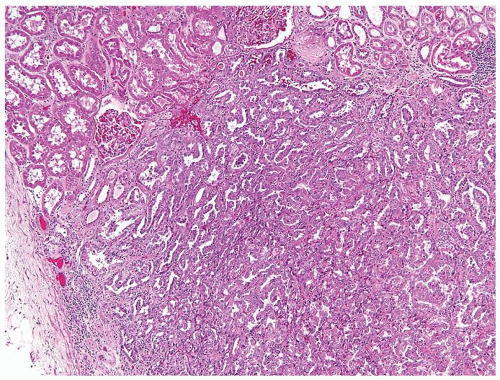
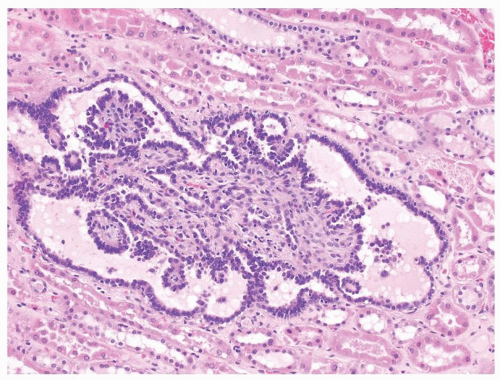
Variable-sized cysts lined by cuboidal clear cells. Septae and wall of cysts contain sheets of cells with expansile solid appearance (Figs.2.9.5, 2.9.6, 2.9.7, 2.9.8, 2.9.9, 2.9.10)
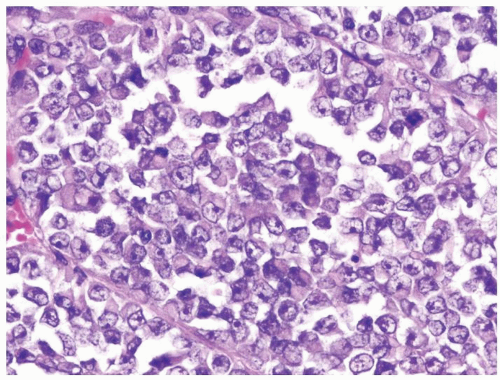
Cytoplasm ranges from optically clear to granular eosinophilic
Nuclear and nucleolar size varies according to tumor
Extrarenal extension uncommon in cystic clear cell RCC
|
Special studies |
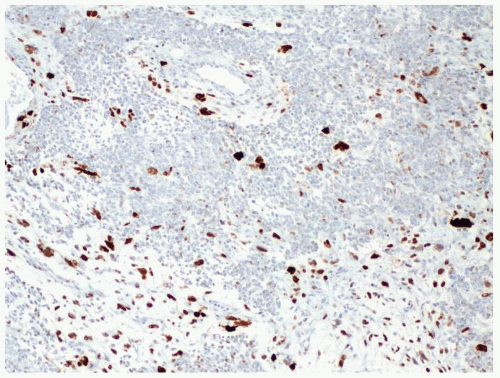
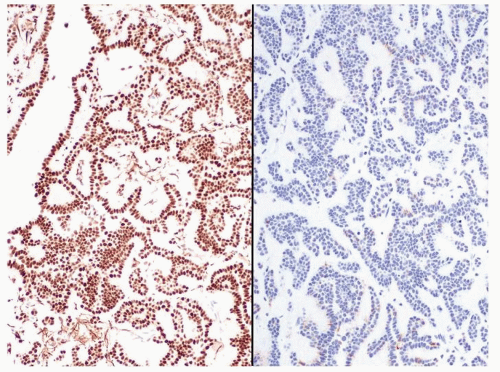
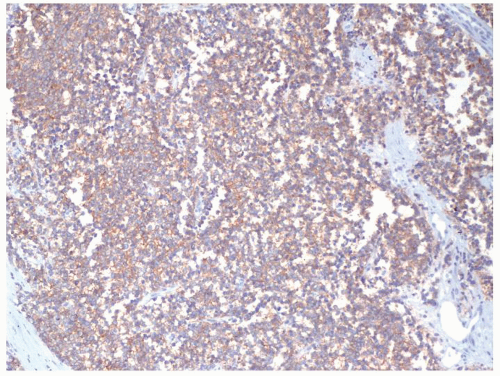
Not helpful in this differential as identical to cystic clear cell RCC |
PAX8, CAIX, RCC, CD10, vimentin, AE1/AE3, positive |
Treatment |
Excision, partial or total nephrectomy |
Partial or radical nephrectomy for localized disease. Targeted therapy is increasingly used for metastatic disease including VEGF receptors, tyrosine kinase receptors, and mTOR inhibitors |
Prognosis |
Surgical removal is curative. Cases with >95% cyst formation and rare nonexpansile low-grade clear cell nests in the septae never been shown to exhibit malignant behavior. We diagnose these lesions as “multicystic renal cell neoplasm of low malignant potential” rather than benign given the lack of long-term follow-up data. Also avoids labeling patients as having “carcinoma,” which has psychosocial and financial implications. Others use “multicystic renal cell carcinoma” for these lesions. In cases with >20% solid areas, uniformly accepted as cystic carcinomas. Unclear of the malignant potential of 5%-20% solid areas, but the current convention is to diagnose “RCC with cystic change” with a comment that their prognosis is favorable |
Primarily stage, grade, and clinical performance status dependent |


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access