Ion-Motive Adenosine Triphosphatases
Various ATPases that drive the transport of cations across biologic membranes have been described. The classes of ion-motive ATPases thus far described are listed in the following table and can be classified into multi-subunit and single-or two-subunit types, the F1F0 and V-ATPases representing the former and the P type the latter. The class of ATPases represented by the P glycoproteins or ABC transporters will not be discussed here.
Classes of Ion-Motive ATPases | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
They have cytoplasmic domains that bind ATP and that transduce the energy from ATP hydrolysis into conformational changes, enabling binding of the ion from the cytoplasmic face of the membrane, movement of the ion into the membrane domain, and release of the ion from the exoplasmic face of the membrane. The cytoplasmic domain of the P-type ATPases or the cytoplasmic or intramitochondrial subunits of the V or F1F0 ATPases contain the energy-transduction sequences that transmit the ATP-induced conformational changes via a stalk region to the membrane domain. Their sequence, or that of one or more of their subunits, contains several relatively hydrophobic amino acid clusters of approximately twenty-one amino acids that are membrane inserted. These membrane segments form the transmembrane domain of these pumps and contain the ion-binding site and the ion pathway across the membrane. These segments are arranged as a compact cluster of membrane-inserted segments with varying degrees of tilt. Although the details of the ion transport mechanism of these pumps are still being explored, it is now apparent that alterations in tilt, and perhaps also rotation, of one or more of the membrane segments underlie the ion transport mechanism across the membrane domains.
Multimeric pumps
F1F0 type of ATPases/ATP synthases
The F1F0 type of ATPases/ATP synthases is found in the inner bacterial, chloroplast, and mitochondrial membranes and catalyzes the synthesis of ATP from ADP and inorganic phosphate (Pi) by dissipation of the electrochemical gradient of H+ generated across these membranes by oriented redox pumps. Working in reverse, they are able to pump H+.
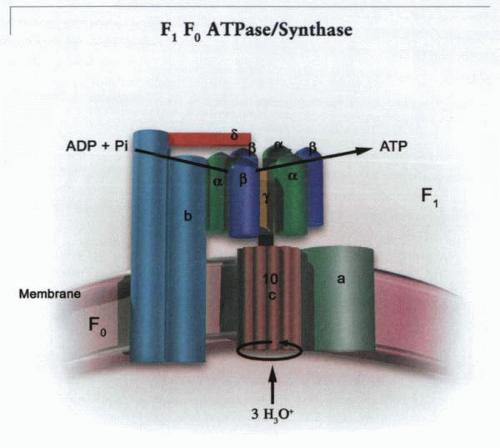
These are multi-subunit pumps with a cytoplasmic domain consisting of a trimer of two subunits (α and β) as well as three other peptides (γ, δ, and ε) connected
to a three-subunit membrane domain (a, b2, c9-12), as shown in the following figure. The γ subunit connects the F0 to the center of the α-β trimer. The remarkable rotary mechanism of this ATP synthase has been deduced by kinetic analysis, cross-linking studies, and high-resolution crystals of the α-β trimer. It is thought that H3O+ traverses part of the membrane domain of the a subunit of F0 driven by the electrochemical gradient for H+. Three or four H+ are then donated to the c subunit (assembled as a nonamer or decamer), initiating a single-step ratchet-like rotation of the c complex. This forces a rotation of the γ subunit, altering the conformation successively of each dimer of the F1 α-β trimeric complex. After this step rotation, the H3O+ is dispersed into the inner mitochondrial space. The two b subunits are attached to the δ subunit that sits on top of the α-β trimer. Hence, the α-β trimer is fixed. The c and the ε subunits are attached to the γ subunit. The c complex (c, γ, ε) is mobile. The γ-subunit rotation results in a relative change in affinity for the binding of ADP and Pi relative to ATP by decreasing the binding affinity of the latter, allowing release of ATP from the α-β monomer. Hence, the driving force for ATP synthesis is cyclic changes in the relative affinities of the various substrates, ATP, ADP, and Pi. Operating in reverse, the synthase acts as an electrogenic proton pump. This is the only cation pump that appears to function physiologically in a reversible manner.
to a three-subunit membrane domain (a, b2, c9-12), as shown in the following figure. The γ subunit connects the F0 to the center of the α-β trimer. The remarkable rotary mechanism of this ATP synthase has been deduced by kinetic analysis, cross-linking studies, and high-resolution crystals of the α-β trimer. It is thought that H3O+ traverses part of the membrane domain of the a subunit of F0 driven by the electrochemical gradient for H+. Three or four H+ are then donated to the c subunit (assembled as a nonamer or decamer), initiating a single-step ratchet-like rotation of the c complex. This forces a rotation of the γ subunit, altering the conformation successively of each dimer of the F1 α-β trimeric complex. After this step rotation, the H3O+ is dispersed into the inner mitochondrial space. The two b subunits are attached to the δ subunit that sits on top of the α-β trimer. Hence, the α-β trimer is fixed. The c and the ε subunits are attached to the γ subunit. The c complex (c, γ, ε) is mobile. The γ-subunit rotation results in a relative change in affinity for the binding of ADP and Pi relative to ATP by decreasing the binding affinity of the latter, allowing release of ATP from the α-β monomer. Hence, the driving force for ATP synthesis is cyclic changes in the relative affinities of the various substrates, ATP, ADP, and Pi. Operating in reverse, the synthase acts as an electrogenic proton pump. This is the only cation pump that appears to function physiologically in a reversible manner.