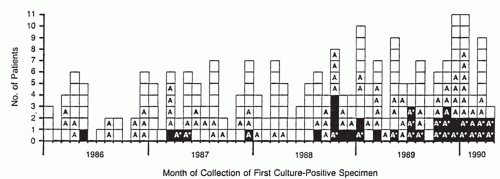
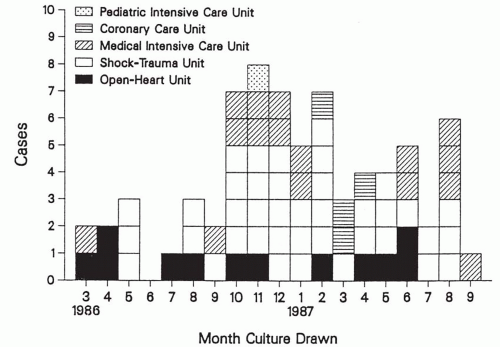
occurs. These clusters merit investigation to determine if HAI transmission really is occurring and to institute appropriate control measures to terminate pathogen transmission. Selection bias frequently occurs in identifying outbreaks because unusual pathogens, or common microorganisms with unusual antimicrobial susceptibility patterns, are more easily recognized. For example, although Escherichia coli urinary tract infection outbreaks probably occur, they are either not recognized or not investigated, because the microorganism is the most common cause of urinary tract infection and typing of the genotying of strains—to document clonal transmission—usually is not performed. In contrast, a small cluster of unusual pathogens or common pathogens with unusual antimicrobial susceptibility patterns are easily and frequently recognized.
specific laboratory or diagnostic findings), epidemiologic parameters (e.g., a patient’s presence on a specific ward or service during a specified time) may be included. In certain instances, one may include confirmed, possible, or probable cases of disease. The process of developing case definitions is an iterative one and should be based on balancing the need for an all-inclusive (sensitive) case definition at the beginning of the investigation and more specific case definition as the investigation proceeds and more data are acquired. Case definitions may vary from the relatively simple to very complex (21,25) (Table 8-2). Occasionally, the case definition may need to be refined as the investigation proceeds and more data are acquired.
TABLE 8-1 Guidelines for Epidemiologic Field Investigations | ||
|---|---|---|
|
TABLE 8-2 Examples of Case Definitions from Hospital Outbreaks Investigated by the CDC’s Hospital Infections Program/Division of Healthcare Quality Promotion | ||
|---|---|---|
|
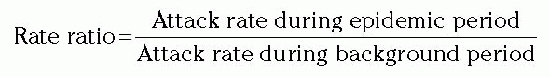
population of patients or HCWs is at risk determines who should be included in further analytic studies.
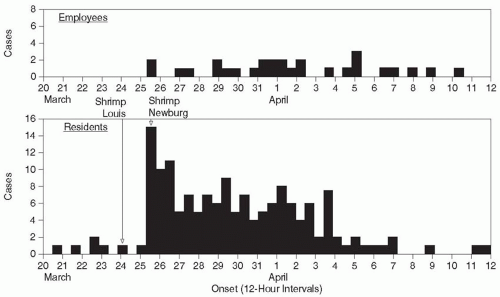
affected by the outbreak, the dates of onset of disease/adverse event for patients and HCWs should be plotted both together and separately to determine if transmission occurred from patient to patient, patient to HCW, HCW to patient, or HCW to HCW.
commonalities identified, and hypothesis generated about the most probable sources and mode of transmission. Then, a variety of control measures are implemented aimed at the most probable source and mode of transmission. After implementing these control measures, one continues to conduct surveillance for additional case-patients and one hopes that the outbreak is terminated. If the outbreak continues, either additional control measures may be implemented or it may be necessary to conduct the hypothesis testing epidemiologic studies.
very useful to direct further analyses. For example, if the study population was exposed to attending physicians A, B, and C as shown in Table 8-3, further analyses might be conducted around events associated with attending-physician A.
TABLE 8-3 Frequency Distribution of Attending Physicians for Cases and Controls, Outbreak of Unknown Disease, Hospital X | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
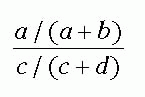
TABLE 8-4 Two-by-Two Table Comparing Physicians A and C to Case-Control Status, Outbreak of Unknown Disease, Hospital Xa | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
associated so that the independent importance of each risk factor cannot be determined. Details on the use of univariate, stratified, and multivariate statistical techniques can be found in Chapter 3.
and Epidemiology, the Society for Healthcare Epidemiology of America, Joint Commission, World Health Organization, or other organizations may lend guidance for specific situations (40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51). If the investigation implicates a particular HCW or item of patient-care equipment, specific measures should be taken to rectify the situation.
blood. A revised hypothesis was that nurse A contaminated the sterile operative field after performing the ACT test; this would account for all of the cases of R. bronchialis SSIs during the epidemic period.
TABLE 8-5 Categorical and Continuous Variables as Measures of Potential Risk for Infection | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
toxicity in hemodialysis patients traced to an aluminum pump (94,103,128), inadequate water disinfection (128), or exhaustion of a reverse osmosis filter (103), respectively; Mycobacterium fortuitum infection or pseudoinfections from inadequate bronchoscopy disinfection (99); Nocardia SSIs traced to a colonized anesthesiologist and his contaminated home environment (102); bloodstream infections traced to needleless devices used in home infusion therapy (107,112,116,125, 126, 127); the role of the nursing shortage on increasing infection rates (106,120); and others. In addition, risk factors for transmission of M. tuberculosis (34,35,74,83,92) to patients and HCWs in healthcare settings were identified, and interventions were implemented and documented to terminate such transmission (54,55,98). Similarly, risk factors for the emergence and transmission of vancomycin-resistant enterococci were identified (104,105,122, 123, 124); then interventions (including active detection and isolation including active surveillance testing and barrier precautions) were implemented and shown to be effective in reducing or eradicating transmission on a ward (104,105), in an entire hospital (124), or in an entire region of a state (all acute care and long-term care facilities) (141). In addition, new and emerging HAI pathogens were identified, such as M. furfur in neonates (57,115), Y. enterocolitica in red blood cell products (58), P. (now Burkolderia) cepacia in cystic fibrosis patients (59,73), multidrug-resistant M. tuberculosis (4,25,34,35,54,55,74,83,92,95,98), nontuberculous mycobacteria in hemodialysis patients (60) or bronchoscopy patients (99), R. bronchialis or Norcardia farcinica in cardiac surgery patients (52,102), Enterobacter hormaechei in neonates (99), Akremonium kiliense in surgical patients (109), Ochrabactrum anthoropi in pediatric patients (114), vancomycin-resistant enterococci (104,105,122, 123, 124,141), and S. aureus with reduced susceptibility to vancomycin (142).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree