Introduction to Kidney Tumors
Satish K. Tickoo, MD
Victor E. Reuter, MD
TERMINOLOGY
Abbreviations
Renal cell carcinoma (RCC), urothelial carcinoma (UC)
EPIDEMIOLOGY
Incidence
RCC accounts for approximately 2% of all cancers
In 2009, there were estimated 57,760 new cases of kidney and renal pelvic cancer in USA
In UK, more than 6,500 cases are reported per year
Incidence of RCC has increased substantially over the last 2 decades
Increased incidence, at least in part, is a result of improved diagnostic techniques
Most cases of RCC in larger medical centers are now incidentally detected, mostly on radiologic investigations for unrelated conditions
Compared to renal cortical tumors, carcinomas of renal pelvis and ureter are relatively uncommon
They constitute 0.1% and 0.07% of all cancers in men and women, respectively, in North America
Account for 4-5% of all urothelial tumors
Majority (> 90%) of these tumors are usual urothelial (transitional cell) carcinomas
The rest are tumors with aberrant histologies, squamous cell carcinomas being the most common of these
Ethnicity Relationship
Incidence varies among countries, with highest rates in North America and Scandinavia
In USA, incidence is equal among whites and blacks
Gender
RCCs and urothelial carcinomas of renal pelvis occur 2x more frequently in men than in women
Natural History
Renal cell tumors
In USA, close to 13,000 deaths due to RCC are reported each year
Worldwide, the disease results in > 100,000 deaths every year
Up to 30% of patients with RCC present with metastatic disease, and recurrence develops in 40% of patients treated for localized tumor
5-year survival rates historically are approximately 40%; median overall survival in patients with metastasis is approximately 12 months
Recently, targeted therapies against various pathway molecules active in RCC have shown promising results
Renal pelvic and ureteric tumors
5-year survival
> 99% for Ta
91% for T1
72% for T2
40% for T3
16% for patients with metastasis
Age Range
RCC and UC of upper tract show wide age spectrum
However, peak incidence in 6th and 7th decades of life
CLINICAL IMPLICATIONS
Anatomic Considerations: Renal Cell Tumors
Gerota fascia (renal fascia)
Layer of connective tissue encapsulating perirenal fat, and the kidney and adrenal within it
Anterior to this fascia is anterior pararenal space, which contains pancreas, transverse colon, and parts of duodenum
Surgeons typically remove the kidney along with its Gerota fascia
Microscopically, Gerota fascia does not have any distinctive features, other than ill-defined, somewhat compressed connective tissue
For practical purposes, tumors present at soft tissue margins of specimen are considered to invade Gerota fascia (pT4)
Protrusion vs. perinephric fat invasion
RCC frequently shows exophytic, often mushroomlike component protruding into perirenal fat
It is usually capped by well-defined smooth fibrous capsule
Unless tumor shows irregular extensions, incomplete pseudocapsule, or single cells invading fat, not regarded as extracapsular extension (pT3a)
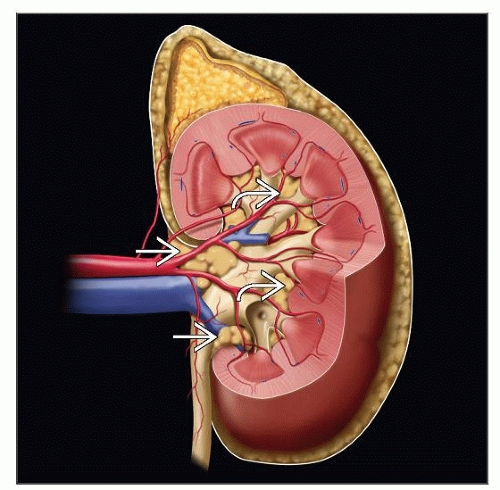
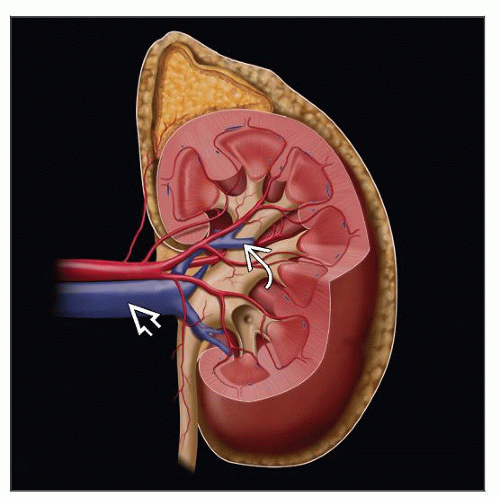
Renal sinus
It constitutes extrarenal soft tissue lateral to imaginary vertical line joining medial-most aspects of upper and lower renal poles
Contains adipose tissue, lymphatics, veins, arteries, nerves, and pelvicalyceal system
Extends deep into kidney, while surrounding calyces (“intrarenal portion of sinus”)
Invasion of sinus fat or sinus veins may occur around pelvis or deep within “intrarenal portion of sinus” (pT3a)
Unlike that in the rest of the organ, the kidney lacks a renal capsule in sinus
Renal sinus vein and fat invasion
According to AJCC/TNM staging, sinus fat or extrarenal fat invasion assigned same pT stage (pT3a)
Similarly, invasion of muscular branches of renal vein in renal sinus and main renal vein invasion also assigned same pT stage (pT3a)
Careful evaluation reveals sinus fat or vein invasion in overwhelming majority of tumors > 7 cm in diameter
Smaller tumors located close to renal sinus also frequently show sinus vein or fat invasion
Current AJCC/TNM staging designates tumors > 10 cm confined to kidney as pT2b
However, probability of such large tumors limited to kidney is low and warrants close gross evaluation and adequate sampling to rule out extrarenal extension
Microscopic presence of large tumor masses in sinus veins, in spite of not being mentioned in gross description, usually suggests inadequate gross evaluation
Presence of intravenous tumor masses on microscopy may be considered equivalent to gross venous involvement not picked up on grossing
Sinus fat invasion may occur as direct tumor extension into fat or tumor present in veins penetrating through vessel wall
Some authors believe that penetration out of venous walls is main mechanism of sinus fat invasion
Anatomic Considerations: Renal Pelvic and Ureteric Tumors
Renal papillae are directly covered by urothelium, without underlying muscularis
Early invasion in area of renal papilla directly involves renal parenchyma (pT3)
On the other hand, invasion in pelvicalyceal system away from renal parenchyma often results in lower pT stage (pT1 or pT2)
Ureter does not contain muscularis mucosae, and muscularis (propria) often extends close to urothelium
Therefore, invasion in ureter more readily involves muscularis propria (pT2)
Intraoperative (Frozen Section) Evaluation: Main Indications
To determine whether the tumor is a renal cortical neoplasm or urothelial carcinoma of pelvicalyceal system
Distinction particularly important when partial nephrectomies are being contemplated
For urothelial carcinoma, partial or even total nephrectomy is usually not adequate or acceptable option
Standard surgical procedure for urothelial carcinoma is nephroureterectomy, ± resection of bladder cuff
For renal cortical neoplasms, no further intraoperative action may be needed
Specific intraoperative subtyping of cortical tumors is not required/necessary, as surgical management is not dependent on specific tumor type
To evaluate surgical margins, particularly in partial nephrectomies
Positive “frozen section” margins will often lead to additional surgical resection for cortical tumors
Staging Issues: Renal Cortical Tumors
Renal cortical tumors confined to kidney assigned stages pT1 or pT2 by AJCC/WHO
Specific maximum size of primary tumors reported as important prognostic factor in many studies, but not always on multivariate analysis
Size as a continuous variable more often shown to have impact on clinical outcome
However, specific size limits are considered useful for purposes of management and clinical trial protocols
Therefore, specific sizes used in TNM staging
Soft tissue or vascular spread beyond kidney (pT3) recognized as major prognostic factor
Before the 6th edition of TNM/AJCC staging system (2002), no mention was made of renal sinus fat or renal vein branch invasion
Multiple recent studies report prognostic significance of renal sinus fat or muscular branches of renal vein invasion
Adrenal gland invasion
Involvement of ipsilateral adrenal gland by direct spread occurs in about 5% of cases
In current AJCC/TNM staging system, it is regarded as stage pT4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree