Intrinsic Factor
Historical issues
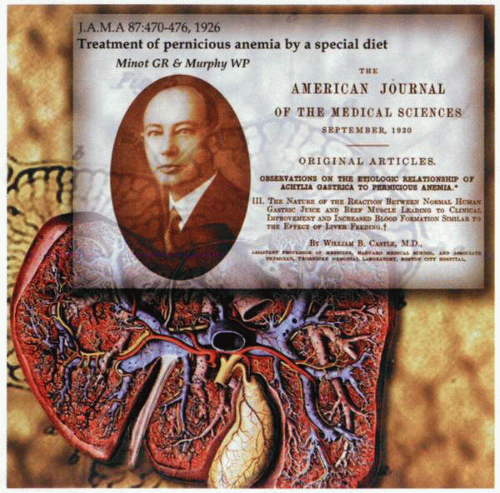
Although pernicious anemia was clearly recognized by Addison in 1855, this fatal anemia did not have any effective treatment until 1926, when Minot and Murphy documented satisfactory management with the ingestion of large amounts of raw liver. A debt of gratitude is owed to Castle, who proposed that impaired gastric function was responsible for the disease and successfully proved his hypothesis. Thus, neither beef (200 g daily) nor normal human gastric juice (150 mL) administered by stomach tube produced a hematopoietic response. Nevertheless, simultaneous administration of both resulted in a positive response as effective as that induced by liver alone. It subsequently became apparent that liver contained high levels of cobalamin, and because intrinsic factor was not needed for the nonspecific diffusion of the vitamin across the mucosal membranes, cure resulted—albeit in an inefficient fashion. The availability of isotopically labeled cyanocobalamin in 1950 allowed for the full understanding of the role of intrinsic factor in the absorption of cobalamin. Further studies enabled the localization of the site of intrinsic factor production to the parietal cell and identified two distinct functional sites on the intrinsic factor molecule. The further identification of ileal membrane receptors for intrinsic factor-bound cobalamin demonstrated a role for the pancreas as well as the stomach in normal cobalamin absorption. In addition to intrinsic factor, it also became apparent that there were other cobalamin-binding proteins involved in the normal transport of the vitamin.
Cobalamin
Initial confusion in the nomenclature of the cyanocobalamin reflected the fact that the vitamin exists as hydroxycobalamin, methylcobalamin, and adenosylcobalamin. Given the fact that all of the above compounds have the biologic activity formally attributed to vitamin B12, the general term cobalamin is now used.
The structure of adenosylcobalamin is a single cobalt atom in the nucleus of a molecule coordinated in the center of a planer corrin ring by the nitrogens of four pyrroline rings. Cobalamin cannot be synthesized by mammalian tissues—only by microorganisms. Thus, a wide variety of bacteria and protozoa are capable of production of cobalamin, and microbial synthesis of the vitamin serves as the ultimate source for the mammalian organism. Mammalian tissues are capable of efficiently converting hydroxycobalamin to the coenzymes methylcobalamin and adenosylcobalamin. It is of considerable importance that the mammalian organism exhibits absolute dependence on the vitamin for normal cellular replication. Despite the fact that the vitamin is crucial for tissue viability, mammalian organisms are not effectively able to assimilate cobalamin by a simple passive process. The relatively large molecule has a radius far in excess of the “effective pore size” of intestinal absorptive membranes. By virtue of its structure, however, it is highly soluble in water and virtually insoluble in most organic solvents. This bulky, polar, water-soluble, lipid-insoluble molecule, therefore, is not easily able to pass the lipid bilayer of biologic membranes and requires a highly specialized membrane-transport system for assimilation and use. Because only 10 to 15 μg per day of cobalamin is available in a diet, the membrane-transport process requires precise identification of the molecule and an effective extraction process. Similarly, transport needs to be specific to avoid absorption of cobalamin-like molecules, which are either inactive or possibly harmful.
The structure of adenosylcobalamin is a single cobalt atom in the nucleus of a molecule coordinated in the center of a planer corrin ring by the nitrogens of four pyrroline rings. Cobalamin cannot be synthesized by mammalian tissues—only by microorganisms. Thus, a wide variety of bacteria and protozoa are capable of production of cobalamin, and microbial synthesis of the vitamin serves as the ultimate source for the mammalian organism. Mammalian tissues are capable of efficiently converting hydroxycobalamin to the coenzymes methylcobalamin and adenosylcobalamin. It is of considerable importance that the mammalian organism exhibits absolute dependence on the vitamin for normal cellular replication. Despite the fact that the vitamin is crucial for tissue viability, mammalian organisms are not effectively able to assimilate cobalamin by a simple passive process. The relatively large molecule has a radius far in excess of the “effective pore size” of intestinal absorptive membranes. By virtue of its structure, however, it is highly soluble in water and virtually insoluble in most organic solvents. This bulky, polar, water-soluble, lipid-insoluble molecule, therefore, is not easily able to pass the lipid bilayer of biologic membranes and requires a highly specialized membrane-transport system for assimilation and use. Because only 10 to 15 μg per day of cobalamin is available in a diet, the membrane-transport process requires precise identification of the molecule and an effective extraction process. Similarly, transport needs to be specific to avoid absorption of cobalamin-like molecules, which are either inactive or possibly harmful.
Cobalamin-binding transport proteins
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree