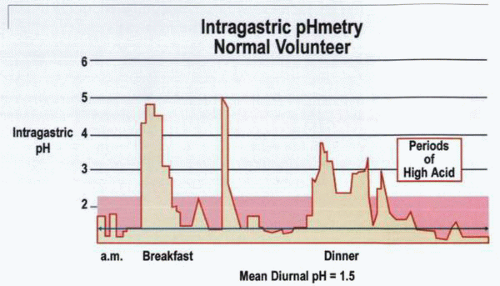
Intragastric PH
Profound acid suppression has raised several concerns about the possible consequences of long-term acid inhibition. These have included the loss of bactericidal activity of gastric acid with an increase in bacterial overgrowth, with the predisposition either to enteric infection or to the increased production of potentially carcinogenic N-nitrosamines and nitrosamides by colonization of the stomach by nitrate-reducing bacteria. Elevation of plasma gastrin has been associated with ECL cell hyperplasia and the possibility of the development of gastric carcinoids. Long-term pharmacologic suppression of acid secretion has been equated to conditions such as pernicious anemia or to situations analogous to those found after subtotal gastrectomy, for which, after 15 to 20 years, an increased risk of gastric cancer has been proposed. To evaluate both the benefits of acid suppression and some of the issues related to the subsequent prolonged elevation in pH and gastrin levels, the measurement of actual gastric acid secretion in addition to gastric luminal pH needs to be considered.
Hypochlorhydria and achlorhydria
A confusing terminology has grown up around the inability to adequately quantify low levels of gastric acid secretion.
In 1886, Chan and Von Mehring associated pernicious anemia with anacidity. In 1889, Einhorn first used the term achylia gastrica to describe the absence of both enzymes and acid in the stomach. Achlorhydria was used to denote the absence of free acid as determined by Topfer’s reagent. Despite these different terminologies, there was no good agreement as to what they meant; nor did they identify whether the conditions that caused them were reversible or irreversible. In addition, achlorhydria suggested that no acid at all was being secreted, whereas in fact, the presence of some parietal cells secreting acid might be obscured by bicarbonate secretion, and, thus, absence of gastric secretion could not be detected by pH measurement alone. In 1952, Card and Sircus proposed that pH of 6.0 be used to define anacidity. A reasonable definition, therefore, of achlorhydria is the persistent failure of intragastric pH to fall below 6.0 in the presence of any stimulation of gastric acid secretion.
 W. I. Card (inset), I. N. Marks, and W. Sircus defined the different levels of acid secretion and their chemical relevance. |
The terms hypochlorhydria and hypoacidity have not achieved wide acceptance, because the pH electrode does not distinguish between HCl and other inorganic or organic acids, and acid secretion varies widely, depending both on the time of measurement and the methodology used.
Quantification of gastric acid secretion
Two approaches have been used to measure gastric acid secretion, one quantitative and the other qualitative. The quantitative measurements have included basal and stimulated acid secretion, nocturnal acid secretion, food-stimulated acid secretion using intragastric titration, and 24-hour acid secretion. The qualitative measurements use 24-hour intragastric acidity. For the most part, quantitative measurements are rarely used today except under experimental circumstances to define physiologic pathways or to evaluate the effect of therapeutic agents on gastric acid secretion. Stimulants include histamine, pentagastrin, insulin, 2-deoxyglucose, or sham feeding. Currently, the use of quantitative acid-secretory measurement is rarely applied except in the diagnosis of the Zollinger-Ellison syndrome and even here has mostly been supplanted by plasma gastrin measurements and the secretin-provocation test.
pHmetric measurement of acid secretion is now the most frequently applied method. This obtains a profile of intragastric acidity and evaluates the state of acid secretion as well as the influence of pharmacologic agents on ulcer healing and symptomatology. Twenty-four-hour intragastric acidity may be evaluated either by repeated rapid sampling of small volumes of gastric juice by aspiration or, alternatively, by continuous recording of intragastric acidity using intragastric pH electrodes. Although the primary agent of interest is acid secretion, the values obtained may also be influenced by bicarbonate secretion, ingested food and fluids, reflux alkaline duodenal juice, and the rate of gastric emptying. Nevertheless, in an individual patient, measurement under such circumstances closely defines the events occurring in that particular stomach.
The measurements obtained have some drawbacks in both qualitative and quantitative groups. Thus, in the aspiration group, pH is measured at fixed time points and fails to define events that might occur in the intervening time. Continuous recording with a pH electrode provides a better overall picture, but the site of the electrode is of critical relevance in determining the pH measurements. Furthermore, because the pH profile varies in different parts of the stomach, not only are sites important, but the maintenance of location throughout the measured period is also important. An advantage of the pH-electrode methodology is its ability to establish the pH in the duodenal bulb, thus quantifying the effects of hypersecretion and the acid load to which the duodenum or a duodenal ulcer is exposed. A critical difficulty in this type of evaluation, however, is stabilizing the pH electrode in the correct position and maintaining it in position for the duration of the study. It has been established, however, that luminal acidity in the duodenal bulb is no higher in ulcer patients than in controls by day or night, and neither fasting nor food produces any difference between these two groups.
In quantitative evaluation of acid secretion, total acid output is the product of the [H+] (millimoles per liter) and the volume of gastric juice secreted (milliliters
per hour). To evaluate acid secretion, the measurements that have been most commonly used include basal acid output (BAO), peak acid output (PAO), and MAO. BAO represents acid secretion in the absence of stimulation, whereas PAO and MAO are measured in response to stimulation, using pentagastrin at a maximal dose and expressed as millimoles per hour. Alterations in gastric acid secretion may be due either to changes in secretory volume or to changes in the acid concentration of the gastric juice secreted, or to both. There is usually a relationship between the volume of secretion and acid concentration, with concentration increasing in parallel with increased volume.
per hour). To evaluate acid secretion, the measurements that have been most commonly used include basal acid output (BAO), peak acid output (PAO), and MAO. BAO represents acid secretion in the absence of stimulation, whereas PAO and MAO are measured in response to stimulation, using pentagastrin at a maximal dose and expressed as millimoles per hour. Alterations in gastric acid secretion may be due either to changes in secretory volume or to changes in the acid concentration of the gastric juice secreted, or to both. There is usually a relationship between the volume of secretion and acid concentration, with concentration increasing in parallel with increased volume.
There is some discussion as to what might be the best methodology for expressing the information provided by pH monitoring. pH is a logarithmic expression of [H+] activity, and therefore, the mean pH is not numerically the same as the mean [H+] converted to pH units. Thus, averages of pH recordings reflect a geometric mean, whereas average concentration-derived data provide only an arithmetic mean. Because the two means do not provide the same value, they are capable of producing different assessments of acid suppression, particularly in the evaluation of acid-suppressive agents.
Thus, reporting that gastric pH may be above or below a particular pH value somewhat marginalizes the information, because an increase in pH of one unit reflects a suppression of acidity of 90%. Similarly, it is difficult to evaluate whether an alteration of pH from 2.0 to 1.0, as opposed to from 4.0 to 3.0, is reasonable, because both are equivalent to 90% acid suppression. Similar problems apply to the expression of the information when using the area under the concentration/time or pH/time curve, and it seems likely that the best compromise may be the use of median pH or hydrogen ion concentration [H+]. Nevertheless, because it is not possible to use all data points, analyses are usually conducted using summary values and particular time points compared to specific activities such as meals, sleeping, and drug ingestion. It




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree