Inhibition of the Histamine-2 Receptor
Histamine antagonists
The pharmaceutical industry had long recognized the importance of peptic ulcer disease, and many companies had intensive programs searching for a treatment. In most cases, they used an animal model, such as the pylorusligated rat, measuring the change in ulceration given by compounds in their chemical libraries. This approach was unsuccessful, because the real target was acid secretion, not the number of ulcers found in these rat stomachs.
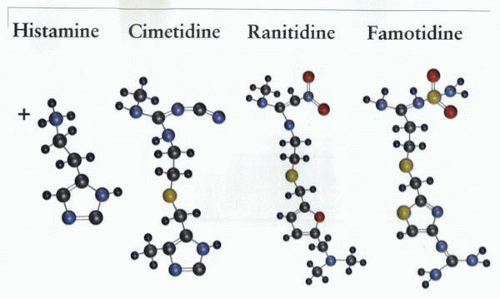
Histamine-1 receptor antagonists were first synthesized by Bovet in the 1940s. Most of these structures were derived by the modification of the imidazole ring of histamine, leaving the ethylamine side chain of the molecule untouched.
These antagonists often penetrate the CNS, resulting in drowsiness. The recognition of the presence of a different subtype of a histamine receptor in the stomach by Keir led to an intensive program for the development of an antagonist selective for what became the H2 receptor. At that time, the histamine-3 receptor had not been discovered, although now it has been cloned. It appears to play an important role in the brain but not in the periphery.
The H2 receptor is found on the parietal cell of the gastric mucosa, where it plays a dominant role in stimulation of acid secretion by binding the histamine released from the ECL cell due to gastrin and PACAP. It is also found in the uterus and in the heart but does not appear to play a medically significant role in these tissues, because these organs remain unaffected by these drugs.
Success in treatment with a receptor antagonist depends on several factors. The expression of several subtypes of receptors for the same ligand differently in different tissues allows the development of receptor- and organ-selective agonists and antagonists. Selectivity is, of course, a paramount requirement for therapy free of side effects.
Lack of side effects depends largely on the distribution of the receptor and its role in the different organs where it is found. Lack of toxicity depends on the nature of the molecule, its metabolism, and its metabolites. Adverse events are idiosyncratic happenings not predictable from preclinical profiles of the
compound; side effects result from lack of target uniqueness and structural problems in the drug.
compound; side effects result from lack of target uniqueness and structural problems in the drug.
Black and his colleagues, after many attempts to modify the imidazole ring to create an H2 receptor-selective antagonist, turned their attention to the side chain and, fairly rapidly thereafter, generated the first H2 receptor antagonist, burimamide. The screen used by this group was inhibition of histamine-induced acid secretion in the perfused rat stomach in the Gosh-Schild preparation, an eminently suitable screen for the discovery of a gastric-targeted antihistaminic! Metiamide and cimetidine followed this first success, and cimetidine was introduced for treatment of acid related diseases in 1977.
The perception of the SK&F research team that the imidazole ring was essential for H2 antagonism allowed relatively simple bypass of the SK&F patent by a group of chemists working at Allen and Hanbury, led by David Jack, also from Glasgow.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree